New therapy holds promise for restoring vision
A new genetic therapy not only helped blind mice regain enough light sensitivity to distinguish flashing from non-flashing lights, but also restored light response to the retinas of dogs, setting the stage for future clinical trials of the therapy in humans.
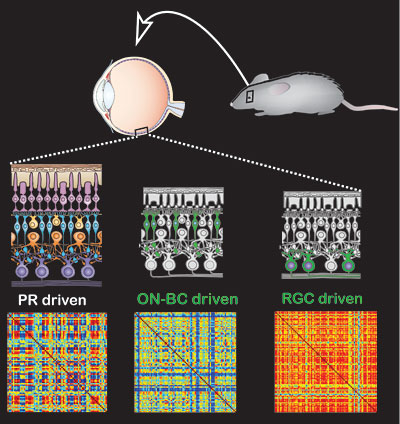
The therapy employs a virus to insert a gene for a common ion channel into normally blind cells of the retina that survive after the light-responsive rod and cone photoreceptor cells die as a result of diseases such as retinitis pigmentosa. Photoswitches – chemicals that change shape when hit with light – are then attached to the ion channels to make them open in response to light, activating the retinal cells and restoring light sensitivity.
Afflicting people of all ages, retinitis pigmentosa causes a gradual loss of vision, akin to losing pixels in a digital camera. Sight is lost from the periphery to the center, usually leaving people with the inability to navigate their surroundings. Some 100,000 Americans suffer from this group of inherited retinal diseases.
In a paper appearing online this week in the early edition of the journalProceedings of the National Academy of Sciences, University of California, Berkeley, scientists who invented the photoswitch therapy and vision researchers at the School of Veterinary Medicine of the University of Pennsylvania (PennVet) report that blind mice regained the ability to navigate a water maze as well as normal mice.
The treatment worked equally well to restore light responses to the degenerated retinas of mice and dogs, indicating that it may be feasible to restore some light sensitivity in blind humans.
“The dog has a retina very similar to ours, much more so than mice, so when you want to bring a visual therapy to the clinic, you want to first show that it works in a large animal model of the disease,” said lead researcher Ehud Isacoff, professor of molecular and cell biology at UC Berkeley. “We’ve now showed that we can deliver the photoswitch and restore light response to the blind retina in the dog as well as in the mouse, and that the treatment has the same sensitivity and speed of response. We can reanimate the dog retina.”
Advantages over other gene therapies
The therapy has several advantages over other sight restoration therapies now under investigation, says vision scientist John Flannery, UC Berkeley professor of vision science and of molecular and cell biology. It uses a virus already approved by the Food and Drug Administration for other genetic therapies in the eye; it delivers an ion channel gene similar to one normally found in humans, unlike others that employ genes from other species; and it can easily be reversed or adjusted by supplying new chemical photoswitches. Dogs with the retinal degeneration provide a key test of the new therapy.
“Our ability to test vision is very, very limited in mice because, even in the healthy state, they are not very visual animals, their behaviors are largely driven by their other senses,” he says. “Dogs have a very sophisticated visual system, and are being used already for testing ophthalmic gene therapy.”

The dogs were chosen because they have inherited a genetic disease caused by the same gene defect as some people with retinitis pigmentosa. Several of them at PennVet were treated and are currently undergoing tests to determine what degree of light sensitivity they now have.
“Seeing that some of the UC Berkeley results with this pharmaco-optogenetic strategy that worked so nicely in mice could be reproduced by our group at PennVet in dogs with late-stage retinal degeneration was really exciting,” said William Beltran, an associate professor of ophthalmology at the UPenn School of Veterinary Medicine. “Use of such a clinically relevant large animal model allows us to begin tackling the next challenges on the road to translating this novel therapeutic strategy to human patients.”
Hybrid chemical-genetic therapy
Genetic diseases like retinitis pigmentosa destroy the photosensitive cells of the eye, the photoreceptors, but often leave intact the other cells in the retina: the bipolar cells that the photoreceptors normally talk to, and the ganglion cells that are the retina’s output to the brain. Isacoff, Flannery and UC Berkeley colleagues have developed several optogenetic techniques for restoring light-sensitivity to surviving retinal cells other than the photoreceptors. These involve using the adeno-associated virus – a common and harmless vector or carrier for gene therapy – to successfully carry a modified gene into these cells. The virus inserts the therapeutic gene into the cell’s DNA and uses its instructions to produce a receptor protein – a modified version of a common glutamate receptor ion channel – that they display on their surface.
The researchers then inject a chemical photoswitch into the eye, “basically, a glutamate dangling on a light-sensitive string,” said Isacoff, “which anchors to the modified receptor and stuffs the glutamate into its docking site on the receptor when activated by light.” The newest version of the photoswitch is fast enough to turn the activity of retinal neurons on and off at a rate that approaches video rate of 30 frames per second.
In mice, they can successfully insert the gene into almost every one of the million or so retinal ganglion cells. This, the researchers say, should restore useful vision.
“So we have reasonable speed and a lot of pixels, now the question is: What can the treated animals see? So far we can say that the treated mice can distinguish between steady light and flashing light. Our next step is to figure out how good they are at telling images apart,” said Isacoff, who holds the Class of 1933 chair.
Which cells get gift of sight?
One key question the researchers wanted to answer is whether it is best to insert photoswitches into ganglion cells or bipolar cells. Viruses can be made to target one or the other. Because activity flowing from upstream bipolar cells to the retina’s output ganglion cells undergoes a lot of processing in the retinal circuit, the researchers were hoping that this same processing would occur when bipolar cells were given a new function they never had before, light-sensitivity.
NIH funding keeps giving
The work was funded in part by a nine-year NIH grant for the Nanomedicine Development Center for the Optical Control of Biological Function.
“The NIH funding got us all the way from designing the chemical photoswitch to an experimental therapy in the dog,” Flannery said, noting the essential role played by a UC Berkeley interdisciplinary team of chemists, molecular biologists and vision scientists.
“And along the way, we developed tools that could be applied to the basic science of how synapses work and how neural circuits work,” Isacoff added. “These are spinoffs that themselves could have implications for the clinic.”
These tools are now the basis of new UC Berkeley projects recently funded by NIH and NSF through President Obama’s BRAIN Initiative.
The answer seems to be yes.
“When we put the photoswitched channels into bipolar cells and record the output of the ganglion cells, we see complicated patterns that look a lot like the activity you get in a normal retina, compared to the on-off activity you get when you put the same photoswitch into a ganglion cell,” Isacoff said.
“The dogs’ behavior should show us if there is a functional difference between driving the system from the bipolar cells versus the ganglion cells,” Flannery said.
He notes that the therapy works only for about a week after a single “charging” with the photoswitch, because the protein and attached chemical get recycled by the cell. While the modified receptors are replaced continually, since the new gene remains forever in the DNA, the chemical photoswitch – maleimide-azobenzene-glutamate, or MAG – must be resupplied by injection into the eyeball. Right now this means injection every week or so, with the future development of a slow release formulation less often.
“This is not necessarily a disadvantage,” Isacoff said, “because the therapy can be stopped, and new photo-sensitive chemicals can be tried as they are improved.”
The researchers continue to study the effects of treatment in both mice and dogs, improve the photoswitch, and develop ways of attaching the photoswitch to other receptors, including some that could amplify the signal and allow perception of fainter light, as occurs normally in rods and cones.
The experiments, analysis and much of the design of the study were performed by first co-authors Benjamin Gaub, a graduate student, and Michael Berry, a technician, along with postdoctoral fellows Michael Keinzler and Andreas Reiner and technician Amy Holt, all from UC Berkeley, and Natalia Dolgova and Sergei Nikonov in the labs of Gustavo Aguirre and William Beltran of UPenn.
The work was funded by a nine-year grant from the National Institutes of Health for the Nanomedicine Development Center for the Optical Control of Biological Function and by a grant from the Foundation Fighting Blindness, USA.
RELATED INFORMATION
- Research profile for Ehud Isacoff
- Research profile for John Flannery
- Restoration of visual function by expression of a light-gated mammalian ion channel in retinal ganglion cells or ON-bipolar cells (PNAS Early Edition)
- NIH awards UC Berkeley $7.2 million to advance brain initiative (9/30/14)
- Shining light on brain circuits to study learning, memory (9/5/14)
- Photoswitches could restore sight to blind retinas (10/31/06)
