Research Bio
The Landry Lab's work develops synthetic bio-mimetic nanocomposites to impart control over nanomaterial interactions with biological systems for two applications: 1) to exploit the intrinsic nanomaterial infrared fluorescence for molecular imaging, and 2) to exploit the highly tunable chemical and physical properties of nanomaterials for targeted delivery of biological cargoes.
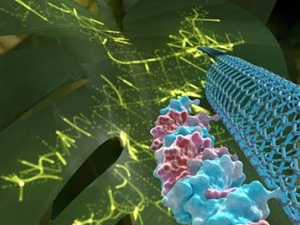
Molecular Imaging: The importance of monitoring neurotransmission is relevant to our understanding of neuropsychiatric disease, in which ‘neurochemical imbalances’ are at the core of many psychiatric and neurodegenerative disorders such as depression, anxiety, Alzheimer’s disease, Parkinson’s disease, and social autism spectrum disorder. Her lab develops optical probes for neuromodulators such as dopamine, serotonin, and norepinephrine. Aberrant neuromodulator signaling in the brain has been implicated in various psychiatric and neurodegenerative disorders, whereby many drugs target neuromodulator signaling. They implement their optical probes for neuromodulators such as dopamine (Beyene et al. Science Advances 2019) and serotonin (Jeong et al. Science Advances 2019) to study addiction, Huntington's Disease, and Parkinson's Disease, among others.
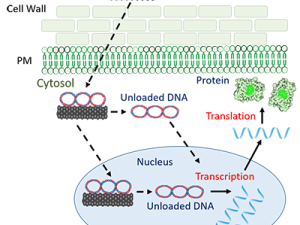
Targeted bio-delivery: The Landry Lab also develops nanoscale particles that enable grafting and delivery of biomolecular cargoes (DNA, RNA, protein) to plants. Despite the importance of creating transgenic plants, plant systems remain difficult to genetically transform because of the unique presence of the plant cell wall, which poses the dominant barrier to exogenous biomolecule delivery. The lab develops nanoparticle-based platforms to enable delivery of gene vectors and RNA (Demirer et al. Nature Nanotechnology 2019), and RNA (Zhang et al. PNAS 2019; Demirer et al. Science Advances 2020; Goh, Zhang et al. Nature Nanotechnology 2021) to agriculturally-relevant plants such as arugula, wheat, and cotton. Biomolecule delivery to plants can assist in plant and crop genetic engineering can solidify the agricultural industry by conferring desirable traits to plants such as increased yield, abiotic stress tolerance, and disease and pest resistance. Production of transgenic plants with nanoparticle-based DNA delivery, without trasngene integration (Demirer et al. Nature Protocols 2019; Demirer, Zhang et al. Nature Protocols 2020), can address the need for sustainable and high-yielding crops without the need to cross out transgenes. Specifically, their platform for delivery of Cas9 DNA vectors represents a platform by which to genetically modify plants without DNA integration (when delivering a DNA vector coding for CRISPR), or without DNA altogether (when delivering an RNP), potentially enabling gene-edited crops to circumvent the process of regulatory oversight as genetically modified organisms (GMOs) (Landry and Mitter, Nature Nanotechnology 2020; Demirer et al. Nature Nanotechnology 2021; Goh et al. Nature Nanotechnology 2022).
Awards and Honors
- 2024 Heising-Simons Faculty Award
- 2024 Notre Dame Thiele Award Lecture
- 2024 Society for Neuroscience (SfN) presidential lecture
- 2023 The Purdue Duncan and Suzanne Mellichamp Award Lectureship
- 2023 Keck Foundation Award
- 2023 Schmidt Polymaths Award
- 2023 Bakar Prize
- 2022 UNC Chapel Hill Distinguished Young Alumni Award
- 2022 Philomathia Prize
- 2022 McKnight Neuroscience Scholar Award
- 2022 Chan-Zuckerberg Biohub Young Investigator
- 2022 Vilcek Prize for Creative Promise in Biomedical Science
- 2021 Chan-Zuckerberg Biohub Young Investigator
- 2021 Nature Research Awards for Inspiring Women in Science
- 2021 National Academies of Sciences Standing Committee on Biotechnology Capabilities and National Security
- 2021 Dreyfus Foundation Teacher-Scholar Award
- 2020 Cell Press 100 Most Inspiring Hispanic/Latinx Scientists in America
- 2020 NSF CAREER Award
- 2020 Frontiers of Imaging: CZI Deep Tissue Imaging Award
- 2020 SfN Janett Rosenberg Trubatch Career Development Award
- 2020 University of Illinois Alumni Association Young Alumni Award
- 2020 Emerging Leader in Molecular Spectroscopy Award
- 2020 ECS Nanocarbons Division Young Investigator Award
- 2019 C&EN Talented 12
- 2019 Bakar Fellow
- 2019 Prytanean Faculty Award
- 2018 Society of Hispanic Professional Engineers Young Investigator Award
- 2018 DARPA Young Faculty Award
- 2018 Sloan Foundation Fellow
- 2017 Kavli Fellow, National Academies of Engineering FOE
- 2017 FFAR New Innovator Award
- 2017 Stanley Fahn Junior Faculty Award
- 2017 Chan-Zuckerberg Biohub Young Investigator
- 2016 Beckman Foundation Young Investigator
- 2016 Burroughs Wellcome Fund Career Award at the Scientific Interface (CASI)
- 2015 Brain and Behavior Foundation Young Investigator Award (NARSAD)
Research Expertise and Interest
nanomaterials, fluorescence microscopy, sensors, imaging, neuroscience, plant engineering