Getting the Lead Out of Drinking Water
Most of us drink water from the tap in our home without a second thought. But for millions of Americans there’s a risk. The United States is facing a serious public health problem from lead contamination of drinking water, especially in rural areas and large cities where efforts to replace aging water service lines has lagged. The problem is especially acute for young children as studies by the Environmental Protection Agency and the Center for Disease Control have linked even small levels of lead exposure to central and peripheral nervous system damage, learning disabilities, shorter stature, impaired hearing, and blood cell disorders.

The most common sources of lead in drinking water are pipes, faucets and plumbing fixtures. Given the challenges, time and expense of renovating water service infrastructures, the most expedient remedy is to install in households point-of-use filters that can remove lead and other heavy metals from water. Such filters offer flexibility, easy operation and cost-effectiveness. However, materials that provide ultrahigh affinity and selectivity for lead ions have been lacking.
Bakar Fellow Baoxia Mi, an associate professor in the Civil and Environmental Engineering Department, and director of research and educational activities at the Membrane Innovation Laboratory, believes she and her research group have found a solution. An expert on advanced membrane processes and nanotechnology, Mi has focused her research on addressing the challenges to maintaining a healthy and sustainable water supply, including in addition to drinking water quality, desalination, wastewater reuse, renewable energy production, and public health protection.
Most recently, Mi and her group discovered that two-dimensional sheets only a few nanometers thick of molybdenum disulfide (MoS2), a semiconductor highly touted for its potential in energy storage and electronic devices, shows the highest lead removal capabilities of any material ever reported. They’re now developing a point-of-use water filter based on stacked layers of MoS2 nanosheets that could provide households with affordable, super-effective protection from lead contamination as well as from other dangerous heavy metals.
Q: Why did you decide to focus your research on issues pertaining to drinking water and what prompted you to test MoS2 as a potential filtering material?
A: My academic training is in water quality engineering, so naturally I’m interested in any technology that may improve the quality of our drinking water. I became interested in using MoS2 as a filter material after learning that it provides strong binding to a number of heavy metal ions including lead.
Q: How does your MoS2 water filter compare to existing point-of-use filtration systems?
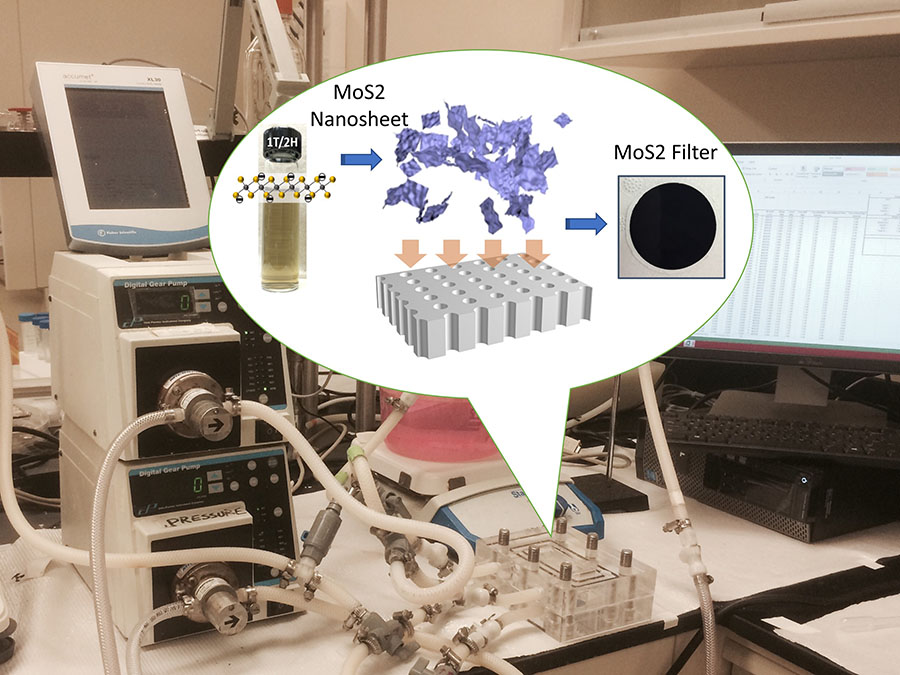
A: We’ve demonstrated that stacking layers of MoS2 nanosheets enables our filter to outperform all commercially available point-of-use filtration systems by a wide margin. Our filter has a much higher lead adsorption capacity and is much more selective for lead over other ions common to drinking water. In addition, a 500 nanometer thick MoS2 filter allows for a high water flow rate while effectively reducing lead concentration in drinking water a thousand fold, from parts per million to less than 10 parts per billion.
Q:What makes your filter so much more effective than other systems?
A: Our filter maintains a stable spacing of approximately 1.2 nanometers between each layer of MoS2. This yields an ultrahigh surface area that exposes all of the sulfur atoms on each nanosheet as accessible lead-binding sites.
Q: In addition to high lead adsorption capacity and selectivity, does your MoS2 filter hold other advantages over current point-of-use filters?
A: Yes, low energy costs, affordability, and convenience are among the most salient of additional advantages. Our MoS2 filter reduces energy demands by delivering a high rate of water flux at low pressure. The material cost is low and the filter can be repeatedly used through regeneration with little maintenance required.
Q: What is the next step for developing your MoS2 filter?
A: First we’ll construct a prototype with the capability of filtering 10 liters (about 2.6 gallons) of water per hour. This prototype will be tested over a full spectrum of tap water chemistry, and its MoS2 layers will be regenerated for repeated use of the filer. We’ll also create a model to evaluate the tradeoffs of key design and operation parameters. Over the next couple of years we plan to evaluate the performance of the prototype in filtering real tap water and wastewater with added heavy metals to mimic contamination events. The water production rate, heavy metal removal efficiency, membrane fouling, and other key operational parameters will be continuously monitored for at least two months, and the lifetime of the device will be estimated based on these results.
Q: How will your Bakar Spark Fund be used in this effort?
A: Our Bakar Spark funding will allow us to more thoroughly evaluate the performance of our filter and tackle the key challenges of scaling up the technology for the commercial market.
The Bakar Fellows Program supports innovative research by early career faculty at UC Berkeley with a special focus on projects that hold commercial promise. For more information, see http://bakarfellows.berkeley.edu.
