Research Bio
Richard Kramer is a Professor in the Department of Neuroscience. His research includes:
Photochemical tools for understanding and re-animating neural signaling in retinal degeneration
The nervous system uses action potentials to send signals over long distances within a neuron and synaptic transmission to send signal across the tiny gap from neurons to other neurons or muscles. Voltage-gated ion channels are crucial for action potentials. Chemical neurotransmitters and their receptors are crucial for synaptic transmission We have developed photochemical tools, called photoswitches, that attach to genetically-targeted ion channels or neurotransmitter receptors to enable optical remote control of these proteins with specificity, and high spatial and temporal precision. Photoswitches open a new window into understanding the specific functions of individual signaling proteins in the living nervous system and are viable drug candidates for neurological disorders, including neurodegenerative diseases that can result in blindness.
Current Projects
Optical control of specific ion channels and neurotransmitter receptors
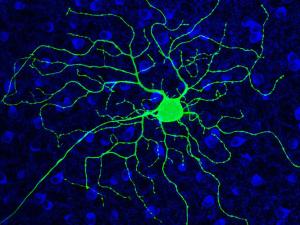
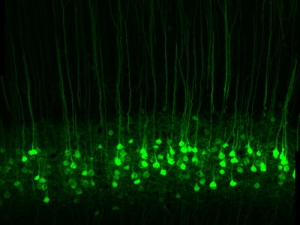
His research group is currently using photoswitches that act on voltage-gated K+ channels, nicotinic acetylcholine receptors, and GABAA receptors. They have generated knock-in mice with a photoswitch attachment site built-in to these specific channels and receptors, enabling photocontrol of the endogenous proteins. With this molecular toolkit, they are addressing questions at various levels of organization in the nervous system, from individual neurons in brain slices to the intact brain of awake, behaving mice. They are interested in the roles that channels and receptors play in neural development, sensory information processing, neural plasticity, and learning and memory. Their studies have extended to many parts of the nervous system, including nociceptive neurons in dorsal root ganglia, neurons in the retina, and interneurons and pyramidal neurons in the hippocampus, cerebellum, and cerebral cortex.
Translating photoswitches into drugs for re-animating vision in the blind
In degenerative blinding diseases such as Retinitis Pigmentosa (RP) and Age-Related Macular Degeneration (AMD), the rod and cone photoreceptor cells die, but the downstream neurons in the retina survive. Most importantly, the retinal ganglion cells (RGCs), whose axons project through the optic nerve to the brain, remain intact. They have developed photoswitches that impart light-sensitivity directly onto native ion channels in RGCs, bypassing the missing rods and cones and restoring the retina’s ability to respond to light and send visual signals to the brain. Their ultimate goal is to translate photoswitch molecules into a vision-restoring drug treatment for blind people. They are testing the effectiveness of photoswitches with three approaches:
- Whole-cell patch clamp and multi-electrode array recordings from the isolated retina,
- Optical Ca2+ imaging of the retina in the intact eye of living mice,
- Behavioral experiments to evaluate restored visual perception.
Reversing pathological changes in retinal neurons to minimize vision impairment
In many patients with RP or AMD, vision is seriously impaired but not completely lost. In these people, the visual deficit is a consequence not only of the loss of signal from photoreceptors, but also the rise of noisy interference from downstream retinal neurons. For example, RGCs become hyperactive, obscuring residual signals generated by surviving photoreceptors and corrupting visual information sent to the brain. His research group recently discovered that retinoic acid (RA) is the molecular trigger of RGC hyperactivity. Hyperactivity is greatly reduced by drugs that inhibit either the synthesis of RA or signaling by RA, a process that involves activation of specific genes. The consequence of these inhibitors is dramatic improvement of vision in mice with a progressive form of RP. Ongoing work is focused on understanding how RA brings about hyperactivity, and developing long-lasting RA inhibitors as treatments for alleviating vision loss.
Research Expertise and Interest
Neurons, synaptic transmission, photopharmacology, ion channels, action potentials, optical imaging, vision, learning and memory, retina, neurodegenrative disease(1551)
In the News
Antabuse May Help Revive Vision in People With Progressive Blinding Disorders
Therapy could improve, prolong sight in those suffering vision loss
NIH awards UC Berkeley $7.2 million to advance brain initiative
The National Institutes of Health today announced its first research grants through President Barack Obama’s BRAIN Initiative, including three awards to the University of California, Berkeley, totaling nearly $7.2 million over three years.
Three Bay Area institutions join forces to seed transformative brain research
Two state-of-the-art research areas – nanotech and optogenetics – were the dominant theme last Thursday, Sept. 18, as six researchers from UC Berkeley, UC San Francisco and Lawrence Berkeley National Laboratory sketched out their teams’ bold plans to jump-start new brain research.
On Memory’s Trail
Ehud Isacoff and his colleagues explore the brain at several levels critical to ultimately understand how memories form and what can threaten their demise. He is the Director of Berkeley’s Helen Wills Neuroscience Institute.
Chemical makes blind mice see
A team of University of California, Berkeley, scientists in collaboration with researchers at the University of Munich and University of Washington, in Seattle, has discovered a chemical that temporarily restores some vision to blind mice, and is working on an improved compound that may someday allow people with degenerative blindness to see again.
Why the eye is better than a camera
The human eye long ago solved a problem common to both digital and film cameras: how to get good contrast in an image while also capturing faint detail. New experiments by UC Berkeley neurobiologists show how the eye achieves this without sacrificing shadow detail.