Research Bio
Professor Somorjai's research interests are in the field of surface science. His group is studying the structure, bonding, and reactivity at solid surfaces on the
molecular scale. This knowledge is then utilized to understand macroscopic surface phenomena adsorption, heterogeneous catalysis, friction, lubrication, and biocompatibility on the molecular level. To this end, research is carried out in the following areas:
a) Studies of the structure and bonding of adsorbed monolayers of molecules on the surfaces of solids by sum frequency generation surface vibrational
spectroscopy, scanning tunneling and atomic force microscopies, and electron spectroscopies. Work is concentrated on transition metal single-crystal and polymer surfaces, and on surface structures of biomolecules (amino acids, peptides) monolayers on these surfaces.
b) Studies of catalytic reactions on single crystals, nano-particles, or other well characterized surfaces. The rate and product distribution of catalyzed reactions are correlated to surface composition, valency, and atomic structure. As part of these studies, we investigate hydrocarbon reactions on platinum, nickel and rhodium.
Most research projects utilize single-crystal surfaces, nano-particles produced by colloid science techniques and as 2-dimensional films or 3-dimensional structures using mezoporous oxides. Extensive use is made of ultrahigh vacuum techniques. Chemical surface reactions are monitored by gas chromatography and mass spectrometry. We always attempt to study the surface on the molecular scale under reaction conditions.
Research Expertise and Interest
physical chemistry, catalysis, surface science, low-energy electron diffraction, solid state chemistry, macroscopic surface phenomena, adhesion, lubrication, biocompatibility, bonding, reactivity at solid surfaces, scanning tunneling
In the News
White House Honors Chemists Hoffman, Somorjai With Enrico Fermi Award
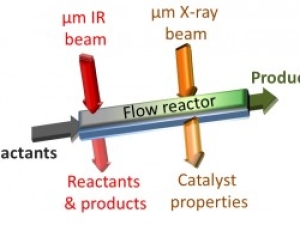
Tracking Catalytic Reactions in Microreactors
Infrared technique at Berkeley Lab’s Advanced Light Source could help improve flow reactor chemistry for pharmaceuticals and other products.
The Best of Both Catalytic Worlds
Catalysts are substances that speed up the rates of chemical reactions without themselves being chemically changed. Industrial catalysts come in two main types – heterogeneous, in which the catalyst is in a different phase from the reactants; and homogeneous, in which catalyst and the reactants are in the same phase.
Chemist Gabor Somorjai awarded frontiers of knowledge prize
University Professor of Chemistry Gabor Somorjai has been awarded the 3rd annual BBVA Foundation Frontiers of Knowledge Award. Accompanied by a $550,000 prize, the award by the Spanish foundation honors Somorjai’s “pioneering experimental and conceptual contributions to the understanding of surface chemistry and catalysis at a microscopic and molecular level.