Tracking Catalytic Reactions in Microreactors
A pathway to more effective and efficient synthesis of pharmaceutical drugs and other flow reactor chemical products has been opened by a study in which for the first time the catalytic reactivity inside a microreactor was mapped in high resolution from start-to-finish. The results not only provided a better understanding of the chemistry behind the catalytic reactions, they also revealed opportunities for optimization, which resulted in better catalytic performances. The study was conducted by a team of scientists with the U.S. Department of Energy (DOE)’s Lawrence Berkeley National Laboratory (Berkeley Lab) and the University of California (UC) Berkeley.
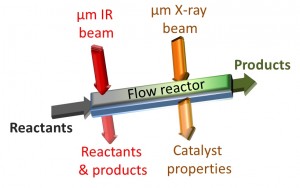
Working at Berkeley Lab’s Advanced Light Source (ALS), the team used tightly focused beams of infrared and x-ray light to track the evolution of a catalytic reaction with a spatial resolution of 15 microns. This research was led by chemists Dean Toste and Gabor Somorjai, both of whom hold joint appointments with Berkeley Lab and UC Berkeley. Somorjai is also a member of the Kavli Energy NanoScience Institute at Berkeley.
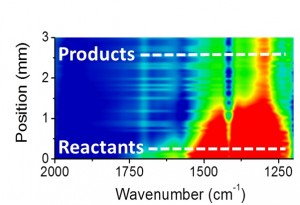
“The formation of different chemical products during the reactions was analyzed using in situ infrared micro-spectroscopy, while the state of the catalyst along the flow reactor was determined using in situ x-ray absorption microspectroscopy,” says Toste, a faculty scientist with Berkeley Lab’s Chemical Sciences Division. “Our results show that using infrared microspectroscopy to monitor the evolution of reactants into a desired product could be an invaluable tool for optimizing pharmaceutical-related synthetic processes that take place in flow reactors.”

Toste and Somorjai are the corresponding authors of a paper in the Journal of the American Chemical Society (JACS) titled “In-situ IR and X-ray high spatial-resolution microspectroscopy measurements of multistep organic transformation in flow microreactor catalyzed by Au nanoclusters.” Elad Gross, a post-doctoral scholar with the corresponding authors, is the lead author. Other co-authors are Xing-Zhong Shu, Selim Alayoglu, Hans Bechtel and Michael Martin.
Catalysts – substances that speed up the rates of chemical reactions without themselves being chemically changed – are used to initiate virtually every manufacturing process that involves chemistry. There are two basic modes of catalytic reactors – batch, in which a final chemical product is produced over a series of separate stages; and flow, in which chemical reactions run in a continuously flowing stream to yield a final product. With the implementation of microreactors, the pharmaceutical industry aims to make the switch from batch mode to flow mode, as flow reactors provide a highly recyclable, scalable and efficient setup that enhances the sustainability and performance of catalysts. However, the synthesis of pharmaceutical drugs is a multiphase, complex process that needs to be carefully monitored. Until now, there has been no capability to follow the multistep production process of pharmaceutical drugs in flow reactors without perturbing the flow reaction.
“Our method allows us to watch an entire catalytic movie, from reactants into products formation, instead of only snapshots of the catalytic process,” says Gross. “In most cases before, chemists had to extrapolate information on the reaction process based on analysis of the final product. With our technique, we don’t have to guess what happened in the first scene based on what we saw in the final scene, since now we’re able to directly watch a high-resolution movie of the entire process.”
For this study, Gross and his colleagues used a heterogeneous catalyst of gold nanoclusters loaded onto a silica support to produce dihydropyran, an organic compound whose formation involves multiple reactant steps. Each of these reactants shows a distinguishable infrared signature, allowing their evolution into the final product to be precisely monitored with an infrared beam. The infrared microspectroscopy was performed at ALS beamline 1.4.
“ALS beamline 1.4 provides a bright infrared beam with a diameter of less than 10 micrometers,” Gross says. “The small diameter of the beam enabled us to draw a map of the flow reactor with high spatial resolution of up to 15 micrometers. Without this high resolution imaging, we would not be able to track and understand key processes in the catalytic reaction.”
In following the reaction kinetics step-by-step, the Berkeley researchers discovered that the catalytic reaction they were observing is completed within the first five-percent of the flow reactor’s volume, which meant that the remaining 95-percent of the reactor, though packed with catalyst did not contribute to the catalytic process.
“Based on this result, we were able to minimize the volume of the flow reactor and the amount of catalyst by an order of magnitude without deteriorating the catalytic reactivity,” Gross says.
While the infrared microspectroscopy technique employed in this study allowed one-dimensional mapping of a catalytic reaction along the path of the flow reactor, the actual flow reactor is three-dimensional. Gross and Toste along with Michael Martin and Hans Bechtel, beam-scientists at the ALS infrared beamline, are now exploring techniques that would permit two- and three-dimensional mapping of catalytic reactions.
“Multidimensional imaging will give us the ability to know where exactly inside the volume of the flow reactor the catalytic reaction takes place,” Gross says. “This will provide us advanced tools for better understanding and optimization of the catalytic reaction.”
This research was supported by the DOE Office of Science.
Additional Information
For more about the research of Dean Toste go here.
# # #
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
The Advanced Light Source is a third-generation synchrotron light source producing light in the x-ray region of the spectrum that is a billion times brighter than the sun. A DOE national user facility, the ALS attracts scientists from around the world and supports its users in doing outstanding science in a safe environment. For more information visit www-als.lbl.gov.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit the Office of Science website at science.energy.gov/.
