Research Bio
Gold Catalysis
Research efforts in the Toste Group have profoundly impacted the development of gold(I)-catalyzed reactions. They enumerated the concept of addition/back-donation that is now heavily employed as a method to generate gold-carbenoid reactivity from alkynes and they described the dual activation paradigm that forms the basis many gold-catalyzed reactions currently being explored by many groups. They have played a leading role in the development of enantioselective catalysis with gold(I) and were among the first to employ gold complexes as catalysts for the oxidative functionalization reactions. They demonstrated that reductive elimination from diarylgold(III) complexes can be remarkably fast and subsequently applied their findings to a non-oxidative gold-catalyzed cross-coupling reaction. More recently, they reported the oxidative addition of gold(I) to strained carbon-carbon bonds giving tunable, stable gold(III) complexes. Moreover, during the exploration of reductive elimination of trifluoromethylgold(III) complexes, they uncovered a new mechanism for the formation of trifluoromethyl-carbon bonds that allows for the introduction of an 18F-radiolabel
Chiral Anion Catalysis
In 2007, they reported the first highly enantioselective transition metal-catalyzed reaction in which a chiral anion was solely responsible for the enantioinduction. This concept was subsequently applied to an enantioselective addition to cationic intermediates. Building on these concepts, in 2011, they applied chiral anions to the first highly enantioselective fluorination of alkenes. In this work, they illustrated a conceptually novel approach to asymmetric catalysis using anionic solid-to-liquid phase transfer catalysis. They have also developed a conceptually novel approach for the enantioselective functionalization of carbon-carbon π-bonds using chiral dithiophosphoric acids as Brønsted acid catalysts. This approach provides the first examples of enantiocontrol of Markovnikov addition to olefins without the use transition metal catalysts. Additionally, they established a collaboration with the Sigman group (University of Utah) to use these reactions as a launching point for our work in data driven discovery of catalysts and reactions.
Supramolecular Catalysis
In collaboration with Profs. Raymond and Bergman (UC Berkeley) , they have demonstrated that reactions can be carried out in water (and accelerated) by encapsulation of the catalyst in an anionic M4L6-type supramolecular assembly (ref 4a). They have also shown that encapsulation increases the stability of the catalysts in the presence of biological catalysts and therefore provides a strategy for carrying out tandem gold-enzyme catalyzed processes. Additionally, they have shown that supramolecular catalysis can accelerate elementary steps important in homogeneous catalysis and showed for the that these two types of catalysis can be combined to generate new reactivity. More recently, they have demonstrated that catalysis in the supramolecular host confers unique selectivity on traditional homogeneous catalyzed reactions, such as hydrogenation.
Heterogeneous Catalysis
In 2010, in collaboration with the Somorjai group (UC Berkeley) , they reported one of the first examples of in situ modification of heterogeneous transition catalyst to provide electrophilic heterogeneous catalysts for the addition of nucleophiles. They have gone onto develop tools for the in situ study of these and other heterogeneous catalysts, including a collaboration with Elad Gross (Hebrew University) developing AFM-IR for studying heterogeneous catalysis. They have also integrated heterogeneous catalyst with biological catalysts to generate process for the conversion of biomass to fuels and chemicals.
Bioconjugation
They are interested in the development of catalysts and reagents for the selective functionalization of complex biological molecules. To this end, they collaborated with the Chang group (UC Berkeley) to develop a reagent for selective functionalization of methionine residues in proteins. They have applied these reactions to the formation of antibody-drug conjugates (ADCs) and to proteomics.
Research Expertise and Interest
organometallic chemistry, organic, development of new synthetic methods, enantioselective catalysts, strategies for the synthesis of natural products, synthesis of complex molecules, formation of carbon-carbon and carbon-heteroatom bonds, olefins, supramolecular chemistry, redox flow batteries, medicinal chemistry, bioconjugation, homogeneous catalysis, heterogeneous catalysis, biomass, fluorination
In the News
National Academy, Royal Society elect new UC Berkeley members
Leaving on a Biofueled Jet Plane
The problem is simple to understand. Molecules of carbon and other greenhouse gases absorb heat. The more greenhouse gases emitted into the atmosphere, the warmer the atmosphere becomes, exacerbating global climate change. Solving the problem is not so simple, especially with regards to aviation – the source of two-percent of the annual greenhouse gas emissions from human activity.
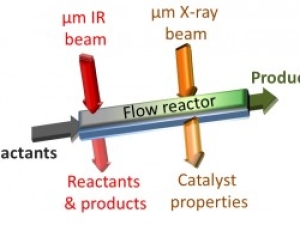
Tracking Catalytic Reactions in Microreactors
Infrared technique at Berkeley Lab’s Advanced Light Source could help improve flow reactor chemistry for pharmaceuticals and other products.
Sweet diesel! Discovery resurrects process to convert sugar directly to diesel
A long-abandoned fermentation process once used to turn starch into explosives can be used to produce renewable diesel fuel to replace the fossil fuels now used in transportation, UC Berkeley scientists have discovered.
The Best of Both Catalytic Worlds
Catalysts are substances that speed up the rates of chemical reactions without themselves being chemically changed. Industrial catalysts come in two main types – heterogeneous, in which the catalyst is in a different phase from the reactants; and homogeneous, in which catalyst and the reactants are in the same phase.