
Research Expertise and Interest
chemistry, inorganic chemistry, neuroscience, bioinorganic chemistry, general physiology, organic chemistry, new chemical tools for biological imaging and proteomics, new metal complexes for energy catalysis and green chemistry, chemical biology
Research Description
Christopher Chang is the Class of 1942 Chair, Professor in the Department of Chemistry and Department of Molecular and Cell Biology, a member of the Helen Wills Neuroscience Institute, and an Adjunct Professor at UCSF.
Chemical Biology, Bioinorganic Chemistry, and Inorganic Chemistry - The Chang laboratory studies the chemistry of biology and energy. They advance new concepts in imaging, proteomics, drug discovery, and catalysis by drawing from core disciplines of inorganic, organic, and biological chemistry. For example, they have developed activity-based sensing as a general platform to identify transition metals, reactive oxygen species, and one-carbon units as new classes of single-atom signals for allosteric regulation of protein function. These chemical tools also reveal unique metal and redox disease vulnerabilities as targets for innovative drug discovery efforts to treat neurodegeneration, cancer, and metabolic disorders. Their work in artificial photosynthesis addresses global challenges in climate change. They use design concepts from biology to develop molecular electrocatalysts for carbon dioxide capture and conversion and nitrogen/phosphorus cycling. Representative project areas are summarized below.
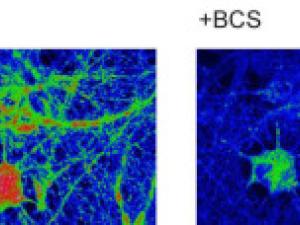
Transition Metal Signaling and Metalloallostery: Bioinorganic Chemistry Beyond Active Sites. They are advancing a new paradigm of transition metal signaling, where metal nutrients like copper and iron can serve as dynamic signals to regulate protein function by metalloallostery, going beyond their traditional roles as static active site cofactors. They develop activity-based sensing probes for imaging mobile transition metal pools and activity-based proteomics probes for identifying allosteric metal sites in proteins. These chemical tools enable us to decipher the complex biology of sleep, cognition, and obesity in cell, zebrafish, and mouse models. They also develop medicines to target metals as disease vulnerabilities in cancer, neurodegeneration, and metabolic liver disorders. These drug discovery efforts focus on cuproplasia and cuproptosis, newly recognized forms of copper-dependent cell proliferation and cell death, respectively.
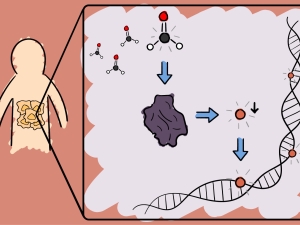
Activity-Based Sensing: Leveraging Selective Chemistry to Decipher New Redox and One-Carbon Biology. They have pioneered the field of activity-based sensing, where they develop chemical sensors for biological analytes that achieve high selectivity using reaction chemistry rather than conventional lock-and-key binding approaches. By applying these chemical tools to enable real-time imaging of reactive oxygen species and one-carbon metabolites at the single-cell, tissue, and animal level, they elucidate principles of how these molecular signals influence fundamental biological processes spanning epigenetics to immune response.
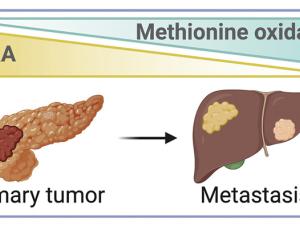
Activity-Based Proteomics: Bioconjugation Chemistry for Single-Atom Signaling and Redox Drug Discovery. They are establishing the area of single-atom signaling, focusing on the study of reversible interconversion of methionine and methionine sulfoxide sites in proteins by adding or removing a single oxygen atom. They develop activity-based proteomics probes to identify new targets of methionine modification as well as writers and erasers that regulate their single-atom biology. These chemical tools also reveal new ligandable hotspots for undruggable protein targets and pathways to accelerate the development of next-generation precision medicines that target redox disease vulnerabilities in cancer and neurodegeneration.
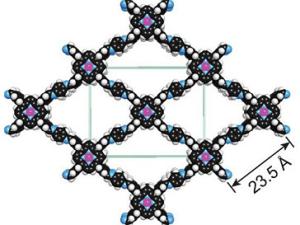
Artificial Photosynthesis: Catalyzing Sustainable Electrosynthesis. They develop catalysts for sustainable electrosynthesis to address climate change and rising global energy demands. Inspired by natural photosynthesis, which produces the value-added products needed to sustain life from light, water, and carbon dioxide, they use biological design principles to create synthetic molecular electrocatalysts for carbon dioxide capture and conversion as well as nitrogen/phosphorus cycling.
In the News
Formaldehyde, a Carcinogen, Is Also Used by the Body To Regulate Our Genes
Scientists Find Trigger That Sets Off Metastasis in Pancreatic Cancer
Guggenheim fellowships awarded to four UC Berkeley faculty
Copper is Key in Burning Fat
A new study is further burnishing copper’s reputation as an essential nutrient for human physiology. A research team has found that copper plays a key role in metabolizing fat.
Copper: A new player in health and disease
Chris Chang, who is part of the Sackler Sabbatical Exchange Program, carries out experiments to find proteins that bind to copper and may influence the storage and burning of fat.
New material captures and converts carbon into useful chemicals
UC Berkeley chemists have taken a promising new material that captures and stores carbon dioxide and altered it to convert the captured carbon into a chemical useful to industry.
Major Advance in Artificial Photosynthesis Poses Win/Win for the Environment
A potentially game-changing breakthrough in artificial photosynthesis has been achieved with the development of a system that can capture carbon dioxide emissions before they are vented into the atmosphere and then, powered by solar energy, convert that carbon dioxide into valuable chemical products.
Copper on the Brain at Rest
A new study shows that proper copper levels are essential to the health of the brain at rest.
Three Bay Area institutions join forces to seed transformative brain research
Two state-of-the-art research areas – nanotech and optogenetics – were the dominant theme last Thursday, Sept. 18, as six researchers from UC Berkeley, UC San Francisco and Lawrence Berkeley National Laboratory sketched out their teams’ bold plans to jump-start new brain research.
Breakthrough in designing cheaper, more efficient catalysts for fuel cells
UC Berkeley chemists Chris Chang, Jeff Long and Marcin Majda have redesigned catalysts in ways that could have a profound impact on the chemical industry as well as on the growing market for hydrogen fuel cell vehicles.