“Intelligent Design” Can It Deliver?
What do Washington lobbyists and gene therapy have in common? Success for both depends on access and influence.
By and large, most drugs are “small molecules,” able to find their way to targets between cells or inside specific cells. They can provide relief from acid reflux, infection or inflammation, or keep serious conditions like diabetes and cardiovascular disease under control. But they demand refill after refill. They aren’t cures.

Serious genetic diseases also require a lifetime of treatment. Bronchodilators and inhaled antibiotics treat severe congestion and lung infections that often shorten the lives of people with cystic fibrosis. Penicillin, blood transfusions and even bone marrow transplants treat sickle cell anemia. A single gene defect causes both conditions.
If the normal gene could somehow replace the functions of the abnormal one in affected cells and tissues, the diseases would be cured. This, of course, is the high ambition of gene therapy. For more than 25 years, researchers have tried the savvy-sounding approach of delivering corrective genes to affected cells by ferrying them inside benign human viruses. Viruses know the territory intimately, so why not piggy back on a proven vehicle?
But, says UC Berkeley bioengineer David Schaffer, “There’s an old joke about gene therapy. ‘The are only three problems in the field: delivery, delivery, delivery.’”
The strategy has run up against a powerful force: evolution. Viruses and the human immune system are locked in a timeless arm’s race. The invader hones its skills, and our immune systems develop antibodies and other defenses to neutralize any innovations. The more persistent the pathogen, the more likely our immune system has encountered it before and has developed antibodies to protect against it.
“We’re fighting biology and evolution,” Schaffer says. The viruses must also reach the right cell type among many within a tissue, and in sufficient numbers to deliver its gene cargo. The challenges have stalled gene therapy from its outset.
Schaffer, director of the Berkeley Stem Cell Center, is a professor of chemical and biomolecular engineering. He was named one of the “Top 100 Innovators under 35” by Technology Review magazine in 2002 for his early efforts to actually use evolution to overcome the hurdles of viral gene delivery. Clean-cut in a checkered shirt, he still looks to be that same young bioengineer. And he has kept his focus on delivering gene therapy.
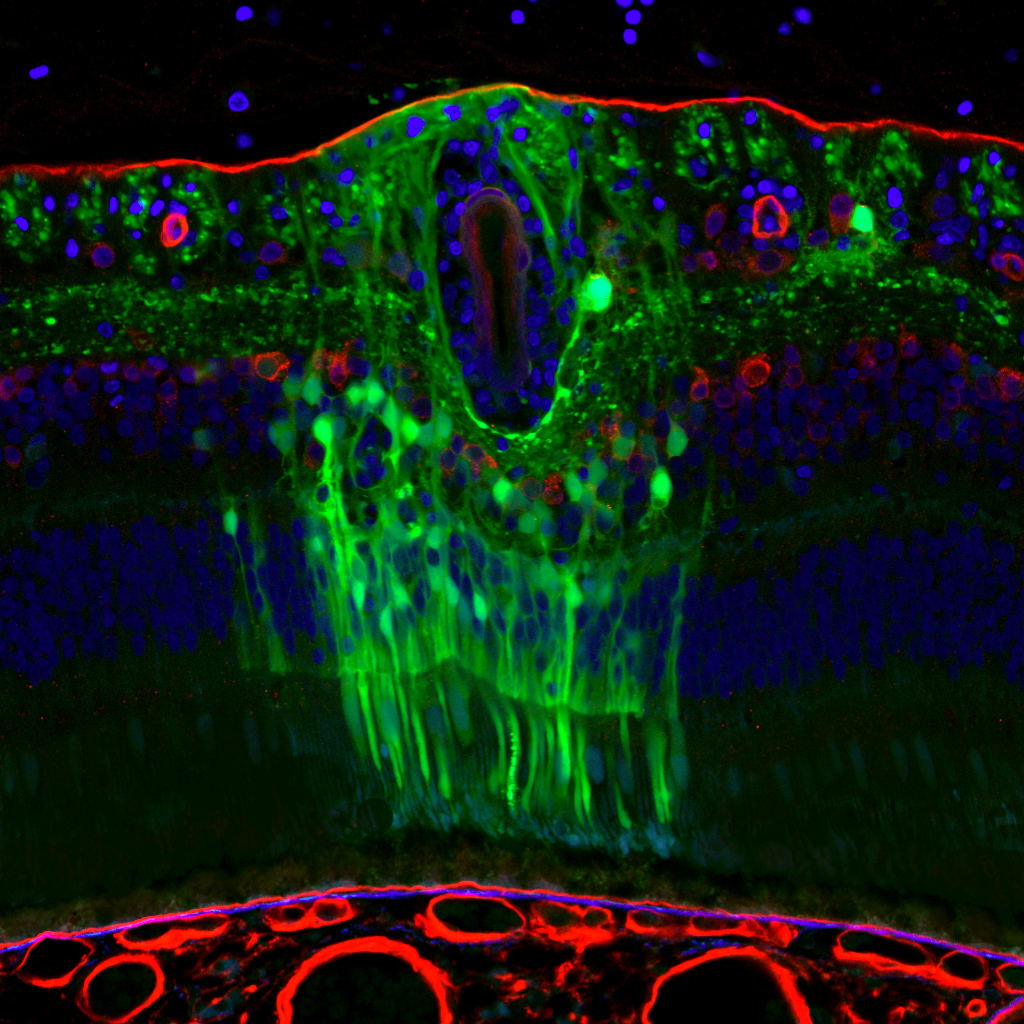
Rather than trying to quiet the body’s defenses against viruses, Schaffer has favored a kind of intelligent design approach to modify the virus. Known as directed evolution, the strategy uses genetic engineering to find variations in the virus that will allow it to effectively deliver drugs to target cells.
His lab works with a common, harmless human respiratory adeno-associated virus, or AAV. More than 90 percent of the human population has been infected by this virus, so it’s got a proven track record for invasion, which our immune systems remember. Schaffer spurs the virus’s evolution in search of a variant that can overcome the immune and tissue defenses.
Our bodies pose many barriers to viral infection, including immune system recognition of the virus’s outer shell, or capsid. In the 1980s researchers discovered that natural variations of a 740-amino-acid-long protein self-assemble to make the AAV capsid. Schaffer’s lab used genetic engineering to shuffle and mutagenize the amino acid sequences and generate “libraries” of millions of variants of the capsid protein.
The team then screened the millions of variants, selecting for the few that could best elude human antibodies, home to the right tissues, and make their way to chosen target cells. The result of this directed evolution: a vehicle that can deliver healthy genes to defective cells.
Last year, he and UC Berkeley colleague John Flannery showed that the virus could deliver curative genes to eye cells to cure several blinding diseases in mouse models. The results show promise for a new way to treat disorders from the inherited defects of retinitis pigmentosa to age-related macular degeneration.
“We’ve created a virus that can deliver genes to a very-difficult-to-reach population of delicate cells in a way that is surgically non-invasive and safe,” Schaffer said at the time. Their success was published in the journal Science Translational Medicine.
His lab also applies the directed evolution strategy to stem cell gene therapy. He and colleagues recently reported using directed evolution of the AAV virus to deliver potentially curative genes to human pluripotent stem cells. “There are many diseases that can be cured by treating stem cells” he says.
The stem cell research field is still young, and the Berkeley Stem Cell Center encourages collaborations between investigators who examine fundamental stem cell properties and bioengineers such as Schaffer who aim to harness the new knowledge.
His success with directed evolution led him and colleagues to launch a start-up last year, called 4D Molecular Therapeutics. They are now working with uniQure, the only company that has a clinically approved gene therapy product. uniQure intends to put 4D’s next generation, evolved viruses into their clinical pipeline.
“The campus really recognizes that translating our research to clinical use is central to its mission to have an impact on society. It’s also a potential source of funds for the campus,” he says. Schaffer sees gene therapy starting out on a track to treat relatively rare diseases for which no other therapies exist. Ultimately though, with effective delivery systems, gene therapy could find its way to treat common diseases involving faulty proteins, such as macular degeneration, diabetes, and heart disease, he says.
That, of course, is well down the line, but Schaffer can see it: “Medicine in the future may involve intelligent viruses that with a single injection can cure a host of diseases.”
