Turkeys inspire smartphone-capable early warning system for toxins
Some may think of turkeys as good for just lunch meat and holiday meals, but bioengineers at UC Berkeley saw inspiration in the big birds for a new type of biosensor that changes color when exposed to chemical vapors. This feature makes the sensors valuable detectors of toxins or airborne pathogens.

Turkey skin, it turns out, can shift from red to blue to white, thanks to bundles of collagen that are interspersed with a dense array of blood vessels. It is this color-shifting characteristic that gives turkeys the name “seven-faced birds” in Japanese and Korean.
The researchers say that spacing between the collagen fibers changes when the blood vessels swell or contract, depending upon whether the bird is excited or angry. The amount of swelling changes the way light waves are scattered and, in turn, alters the colors we see on the bird’s head.
Seung-Wuk Lee, UC Berkeley associate professor of bioengineering, led a research team in mimicking this color-changing ability to create biosensors that can detect volatile chemicals.
“In our lab, we study how light is generated and changes in nature, and then we use what we learn to engineer novel devices,” said Lee, who is also a faculty scientist at the Lawrence Berkeley National Laboratory.
The researchers created a mobile app, the iColour Analyser, to show that a smartphone photo of the sensor’s color bands could be used to help identify toxins of interest. They described their experiments in a study published today (Tuesday, Jan. 21) in the journal Nature Communications.
Sensors that give off color readings are easier to use and read than conventional biosensors. However, the major ones in development elsewhere can only detect a limited range of chemicals and, according to the researchers, they can be very difficult to manufacture.
“Our system is convenient, and it is cheap to make,” said Lee. “We also showed that this technology can be adapted so that smartphones can help analyze the color fingerprint of the target chemical. In the future, we could potentially use this same technology to create a breath test to detect cancer and other diseases.”
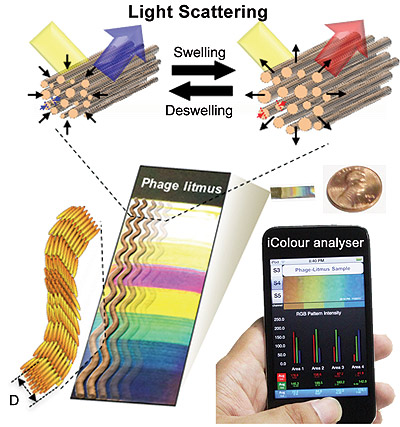
In copying this turkey-skin design, Lee and his team employed a technique they pioneered to mimic nanostructures like collagen fibers. The researchers found a way to get M13 bacteriophages, benign viruses with a shape that closely resembles collagen fibers, to self-assemble into patterns that could be easily fine-tuned.
The researchers found that, like collagen fibers, these phage-bundled nanostructures expanded and contracted, resulting in color changes. The exact mechanism behind the shrinking or expanding phage bundles is still unclear, but it’s possible that the small amount of water in the phage is reacting to the chemical vapors, the researchers said.
The turkey-inspired biosensors were exposed to a range of volatile organic compounds, including hexane, isopropyl alcohol and methanol, as well as vapor of the explosive chemical TNT, at concentrations of 300 parts per billion. The researchers found that the viruses swelled rapidly, resulting in specific color patterns that served as “fingerprints” to distinguish the different chemicals tested.
The researchers showed that the biosensor’s specificity to a target chemical could be increased by genetically engineering the DNA in the M13 bacteriophage to bind with sites specific to TNT. The biosensor was then exposed to two additional chemicals, DNT and MNT, which have similar molecular structures to TNT. The engineered biosensor successfully distinguished TNT from the other chemicals with distinct color bands.
The biosensors were also able to signal changes in relative humidity, ranging from 20 percent to 90 percent, becoming redder with moister air and bluer with drier air.
The study lead author is Jin-Woo Oh, a former postdoctoral researcher in Lee’s lab and now an assistant professor in the Department of Nanomaterial Engineering at Pusan National University in South Korea.
The National Science Foundation, the Defense Acquisition Program Administration and Agency for Defense Development in South Korea, Korea’s Ministry of Education, Science and Technology, and Samsung helped support this work.
