Seeing Through Alzheimer’s Disease
A jury would have to acquit. Two tough guys are caught at the scene of a brutal beating, but no one witnessed the crime. No video cameras or cell phone captured the assault. Maybe both men arrived after the attack. Or one might have acted alone. They’re suspicious, but not guilty beyond a reasonable doubt.
The same might be said about our current understanding of how Alzheimer’s Disease develops. Two proteins — called Beta-amyloid and Tau — definitely muck up neurons in Alzheimer’s victims. Amyloid clumps into plaques that interfere with cell-to-cell communication. Tau proteins contort into tangled fibers inside the cells.
But until recently, neuroscientists have not been able to track the course of Alzheimer’s in the brain. Amyloid and Tau might not destroy neurons directly, or perhaps amyloid works alone, wrecking the delicate integrity of neural networks and degrading memory.
Researchers have been limited to a single snapshot of the brain — one view of the battlefield provided by an autopsy. The course and chronology of the damage that steals memory are still up for grabs.
“We’ve been scratching our heads about how these two proteins are related to each other and to the cause of Alzheimer’s for literally 100 years,” says Berkeley neuroscientist William Jagust. “We don’t know the difference between ‘normal’ memory loss and the likely pathology associated with Tau. We don’t know whether amyloid or Tau is most important in Alzheimers, or if amyloid plaques between neurons affect the Tau tangles inside cells.”
But there’s a sense of anticipation in the air, buoyed by researchers’ increasing ability to peer into the brains of people struggling with Alzheimer’s as well as seniors free of its grip. In the past decade, PET scans and other powerful new imaging tools have begun to fill in the story of how healthy and damaged brains change throughout life.
“We still have many questions and few answers,” Jagust says. “But brain PET scanning in both diseased and healthy people is sort of blowing that wide open. We now have the tools to study the progression of plaque formation from its earliest stages and to determine how amyloid and Tau affect cognitive decline over time.
Before PET scanning, Jagust says, researchers already knew from autopsies that about a third of older people with amyloid plaques had no symptoms of cognitive decline.
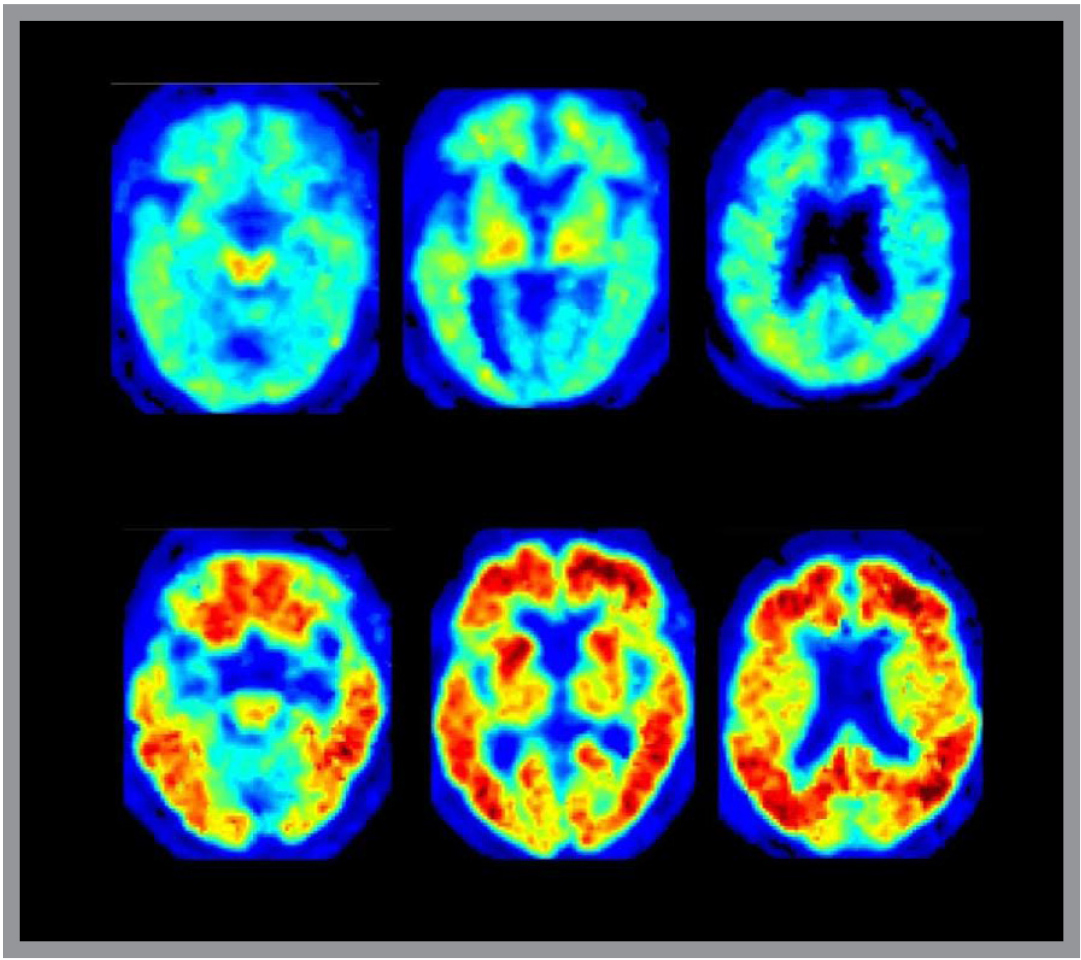
“This created the argument: ‘If they have no symptoms, how can it be that amyloid causes Alzheimer’s?’ You can’t answer that with an autopsy.” But if periodic PET scans show increasing plaque deposition over time as cognitive loss becomes severe, then the plaque argument becomes much stronger.
Research in his lab supports the hypotheses that plaques interfere with the formation or maintenance of synaptic connections. Using the metabolic imaging technique of functional MRI, he focused on plaque-ridden brains of healthy older people, and found indirect, but strong evidence that connections within networks of neurons were breaking down.
“Parts of the brain that should be connected strongly are becoming weakly connected, and parts that are normally not strongly connected become so. It’s almost like the brain is becoming rewired.”
But the rewiring evidence cuts both ways. In another study, Jagust found that some people with amyloid plaques performed as well on memory tests as those who were plaque-free. In some of them, novel connections appeared between neurons in their brains, suggesting new networks were in play.
“There is evidence of ‘rewiring’ that appears to be detrimental, but also evidence of ‘rewiring’ that may serve a compensatory role — providing a cognitive reserve,” he says. “The balance between these in individuals may explain why some decline and others do not.” The research was published in 2013 in the Journal of Neuroscience and in 2014 in Nature Neuroscience.
Several large clinical trials have shown that experimental immunotherapeutic drugs can at least moderately slow Alzheimer’s amyloid plaque deposition. So far, the decline in plaque buildup has not slowed memory loss. But Jagust is confident that the tremendous boost in brain imaging will lead to effective therapies.
One ambitious study, just launched at Harvard and UC San Diego neuroscientists, combines refined imaging and drug trial, focusing on 1,000 people in their 70s and 80s. Study participants do not have Alzheimer’s, though some have amyloid plaques. Researchers hope that by intervening early enough with drugs to slow plaque accumulation, they can prevent or at least delay severe cognitive loss. If early intervention is key, then so is the ability to detect even the slightest sign of neurological damage. The Jagust Lab is using statistical and computational approaches to refine PET scan sensitivity.
In images of people’s brains with significant amyloid deposits, the protein shows up clearly as fiery orange bands across the cerebral cortex, or gray matter areas of the brain. Jagust suspects that improved scanning will allow researchers to spot mere traces that hint at possible trouble to come. The lab is also beginning studies that will image accumulations of tau, allowing the researchers to understand the relationships between tau and amyloid, and provide another target for drug development.
“If the images can tease apart the different roles of Beta-amyloid and Tau, and if we can detect damage in its very earliest stages, we would have strong reason to hope that new drugs can spare or significantly slow cognitive damage. I don’t think this is being overly optimistic.”
In recognition of his research on brain aging and dementia, Jagust received the 2013 Potamkin Prize for Research in Pick’s, Alzheimer’s and Related Diseases by the American Academy of Neurology and the American Brain Foundation.
Additional Information:
Research profile for William Jagust
