Tracking Cancer’s Advance in 3D
Few things are more disturbing than the thought of cancer spreading throughout the body. Intuitively, the best defense would seem to be a direct attack on the rampantly dividing cells. Chemotherapy, for example, homes in on cells that are dividing most rapidly.
But there’s another potential target to stop cancer’s advance — the region between cells that provides essential materiel and routes for cancer’s invasion.

Called the extracellular matrix, or ECM, this natural structural and biochemical support system is under increasing scrutiny for its role in cancer’s advance and as a potential target to block tumor growth. For some types of cancer, this “between-cell” support system may offer the only viable target. This is particularly true for some forms of brain cancer.
Brain tumors known as glioblastomas are extremely hard to treat. They are the most common and most aggressive brain tumors, and patients diagnosed with the disease have a life expectancy of less than two years. It was the cause of Senator Ted Kennedy’s death.
Glioblastoma cells intertwine so tightly with neurons that the diseased brain tissue can’t be excised safely. The brain’s unique makeup also thwarts treatment. In most tissues in the body, collagen provides scaffolding within the extracellular matrix. Cancer cells adhere to it and migrate along its surface. Brain tissue has very little collagen and far less is known about how cancer cells in the brain make their way between cells.
For more than a century, research on cell growth has been carried out in flat, single-layer cell cultures. Mono-layers of cells growing in Petri dishes can be easily observed, teased apart and doused with chemicals in experiments to boost or block growth.
But two-dimensional cultures growing on rigid plastic surfaces have their limits when it comes to mimicking the cells’ natural environment. They can’t provide, for example, the critical three-dimensional structural features in the extracellular matrix that cancer cells use as highways and byways for their advance.
“One of the most important lessons cell biology has taught us over the past several decades is that the structural and mechanical properties of tissue strongly influence cell behavior, especially in cancer,” says Sanjay Kumar, a UC Berkeley expert in the mechanics of cell movement. Researchers have begun to apply the tools of bioengineering to devise materials that model physical features in this extracellular environment. 3D cell cultures are starting to provide a more realistic model of cancer’s movement.
Kumar, professor of bioengineering, has used microfabrication technology to incorporate into living glioblastoma cultures the kinds and textures of proteins and other elements that cancer cells actually encounter.
This year, Kumar is working with colleagues at UCSF’s Brain Tumor Research Center in a collaboration made possible by the Sackler Sabbatical Exchange Program. The program brings UCSF scientists to UC Berkeley and vice versa so the researchers can share techniques and expand collaboration.
Tumor center researchers have access to an unusually diverse range of human tumor cultures. Kumar expects to draw on the resource to improve his models and at the same time, make them readily available to the UCSF scientists.
Kumar’s lab recently fabricated a squishy, 3D material called a hydrogel and incorporated it into cell cultures. This hydrogel closely models the unique connective tissue that serves the same function in the brain that collagen does in most other tissues in the body.
Kumar’s hydrogel is made of large carbohydrate molecules called hyaluronan that are very plentiful in the brain. So, the fabricated material provides both a physical and chemical environment similar to what surrounds cells in the brain.
With this 3D cell culture, Kumar has revealed a unique type of adhesion that glioblastoma cells use to advance and invade. Genetic analysis showed that tumor cells are particularly adapted to make, degrade, and adhere to hyaluronan in the brain. And they adhere to and advance on the lab-made hyaluronan-rich hydrogels.
“The models allow us to understand how and why cells invade the way they do, which is the first step to identifying potential points of attack for new therapies,” Kumar says. “They can also serve as platforms for personalized medicine. One could grow a patient’s tumor in these systems and test various combinations of drugs to determine which ones work best.”
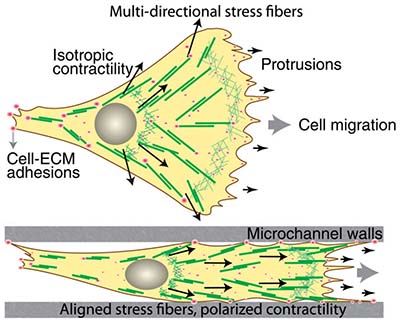
Cancer often progresses along the outside of blood vessels and other elongated structures between cells. In research published in the Proceedings of the National Academy of Sciences, Kumar’s lab microfabricated channels with a stiffness comparable to what is found in brain tissue. The researchers could control the width of the fabricated channels, and they studied how the channel volume affected cancer cell migration.
They showed that, surprisingly, confined channels actually accelerated cell movement through them — presumably because the constriction forces the cells to expend their energy in one dimension rather than exploring two-dimensional space. They also clarified the relationship between channel stiffness and the ease of cell migration.
“Some of this was very surprising to us,” Kumar says. “Our models are extremely simple compared to actual tumors, but we feel that this work can strip away some of the complexity and help us understand what features and mechanics are most critical for driving the growth of these deadly cancers.”
