Molecular Weaving Makes Polymer Composites Stronger Without Compromising Function
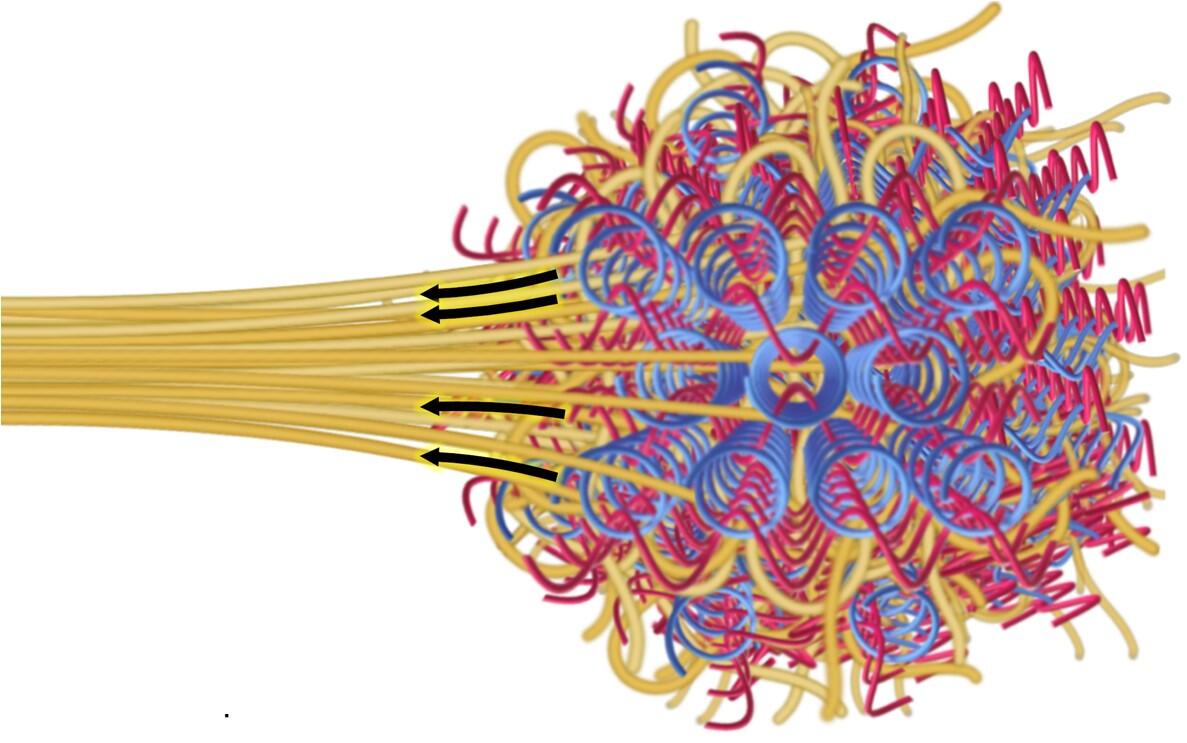
At its most basic, chemistry is a lot like working with building blocks – but the materials are atoms and molecules. COFs – or covalent organic frameworks, a new class of porous crystals – are a great example of a material that behaves like a molecular Lego set, where individual building blocks are connected through strong chemical bonds to form a highly open and structured network. This intricate structure allows them to provide a scaffold for polymer chains to thread or wrap during their formation and strengthen. Think of a woven scarf or basket – a single piece of yarn or twine may not be much on its own, but when woven together, the pattern enhances the overall performance of the final product. Furthermore, when these chains weave together, sometimes even the chemical reactions between them further strengthens the properties of the material itself. In 2016, Yaghi Research Group, led by UC Berkeley’s Professor of Chemistry Omar Yaghi, realized the first molecularly woven structure by interlacing the backbone of the framework in a 3D space. These molecular woven COF crystals are tough but extremely flexible, as every atom has a high degree of freedom to move around but is also locked in place, and as a whole the woven crystals are able to dissipate energy during stress to prevent fracture.
Today, together with Ting Xu, Professor of Chemistry and Materials Science & Engineering; and Rob Ritchie, Professor of Materials Science & Engineering, the lab is now leveraging both the porosity and molecular weaving to make polymer composites stronger, tougher, and more resistant to fracture by threading polymer strands through the woven network. Their findings have been published in a paper by Science.
“This is exciting because most filler materials enhance one mechanical property at the detriment of another,” said Ephraim Neumann, a PhD candidate at the College of Chemistry working at the Yaghi Research Group. Neumann is sharing his first authorship with joint student of Xu and Ritchie, Junpyo (Patrick) Kwon, who graduated (PhD) last year from UC Berkeley.
But why are COFs themselves so useful to everyday life? One example is that due to their exceptional porosity, COFs are used extensively in the storage and separation of gases such as hydrogen and methane. Both hydrogen and methane are clean energy carriers that can be used in fuel cells and combustion engines. Storing them enables their use in transportation and power generation without producing harmful emissions.
Now, thanks to this new research that suggests polymer composites can be made more durable, the applications and uses have wider implications.
“When we add a small amount (1%) of these woven COF crystals to other materials such as polymer or plastic in this case, the materials become significantly tougher and can have a high tolerance for damages and fractures. This could have a huge impact on the materials industry,” said Yaghi.
For example, polyimide, found in almost every laptop and electrical wiring, was one of the investigated polymers in this study. By adding woven COF nanocrystals, the team was able to improve the mechanical performance of the polymer without compromising its thermal stability. This suggests this technique could lead to longer lifetimes for these composites. “Or if the material becomes more resilient, one could use less of it to achieve the same result,” hypothesized Neumann. Polyimide can also be found in the solar sails used by NASA, as it is often used as a support material that lends thermal and mechanical durability to many applications.
“Many properties of plastic products rely on polymer chain entanglements,” said Xu. “My favorite analogy is how an angel hair pasta and a bowtie pasta may respond to a swirl in the plate. Adding nanoparticles of these crystalline COFs can template how these long chains may arrange spatially and get the whole plate to work together. It also becomes feasible to pull out the chains, separate out polymers from COF nanoparticles and do the process again from scratch.”
When thinking about how this might affect industries beyond materials, Neumann concluded, “While this discovery focuses on specific polymers, the basic concept of using porous, molecularly woven COFs to enhance mechanical properties could be extended to many other materials.”
Read the full article on Science Magazine.
