Making molecular movies with X-rays
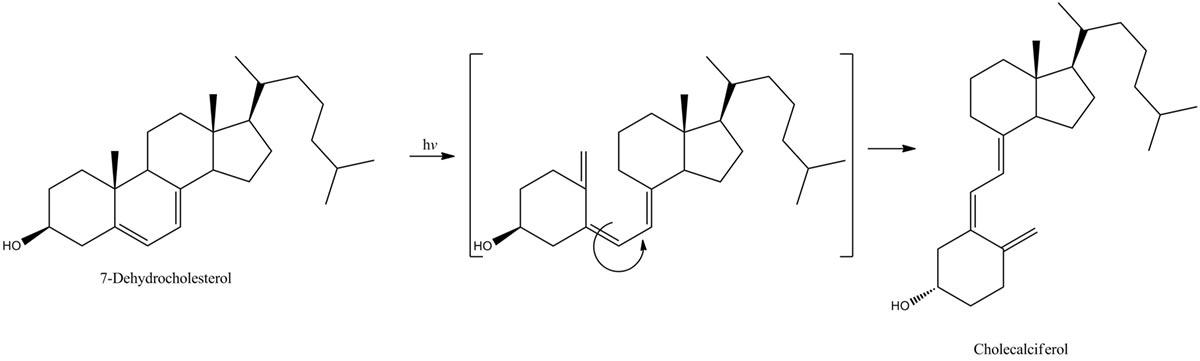
On a sunny day, energetic UVB photons hit a certain type of organic molecule, 7-dehydrocholesterol, in your skin. These molecules absorb the photons, causing a ring-opening reaction that leads to the creation of vitamin D3. If this critical ring-opening reaction didn’t occur, our bodies could not synthesize the vitamin D3 that we need to absorb calcium from our food, transmit nerve signals and keep our immune systems functioning.
Thanks to recent research in the Leone lab, we are gaining new insights into how the ring-opening reaction takes place. These ring-opening or pericyclic reactions are characterized by a cyclic transition state and are common in chemistry. In addition to the photobiological synthesis of vitamin D3, they underlie optoelectronic technologies including optical switching and data storage, photochromic devices and nanomechanical motors.
The obvious difficulty in studying chemical reactions is that molecules and elections are miniscule. But a second problem is that they interact very quickly. Atoms and electrons rearrange on attosecond (10-18 s) to femtosecond (10-15 s) timescales.
In order to better understand such reactions in real time, the Leone lab’s Andrew Attar and colleagues are developing ultrafast spectroscopic devices that not only fit on a table top, but also produce the hard-to-generate UVB photons that allow your skin to make vitamin D3.
“Electrons are the quantum wrapping paper that hold molecules together,” explains Attar. “As a particular reaction reaches its transition state, subtle differences in the arrangement of the electron clouds affect the path the reaction will take. This path in turn affects the three-dimensional shape of the end products and their chemical reactivity.”
“By using extremely short flashes of light,” he adds, “we can capture still frames of chemical processes as they occur. These are then stitched together to create a ‘movie.’ Our x-ray movie is able to directly observe the rearrangement of the electrons within the molecule as the transition state is formed.”
The reaction in Attar’s experiment is triggered by the absorption of ultraviolet light that provides the energy to transform a ring-closed 1,3-cyclohexadiene (CHD) molecule into the ring-opened version, 1,3,5-hexatriene. This occurs on a timescale of about 200 femtoseconds.
The initial absorption of ultraviolet light promotes the electrons of the molecule into a higher-energy arrangement, known as an excited state. This energy is briefly stored as potential energy in the new electron configuration. However, just like a ball at the top of a steep ramp will roll downhill, the potential energy in the electron configuration will be converted to kinetic energy. This corresponds to extremely fast motion of the atoms in CHD. As the atoms start to reorganize, the structure of the electron cloud transforms accordingly, the bonds shift and the ring opens.
To fully understand this interplay between atomic and electronic transformations, the researchers used specific energies of x-ray light to measure the changes in CHD. X-rays are capable of providing tremendously detailed information about both molecular and electronic structure. However, working with X-rays has historically been constrained to large-facility sources like LBNL’s Advance Light Source or other synchrotrons and free electron lasers.
Attar and and coworkers in the Leone group have developed a way to extend these capabilities to the bench top using a laser-based soft X-ray source. With their new ultrafast X-ray source, the researchers are able to characterize and distinguish between the structure of the electron clouds and atomic arrangement in the critically important intermediate of photoexcited CHD that eventually leads to ring opening.
This new instrument is now being utilized to make molecular movies of a myriad of light-activated chemical reactions. These studies promise to expand our understanding of the coupled evolution of molecular and electronic structure which lies at the heart of many chemical reactions.
This research was featured in Science magazine: http://science.sciencemag.org/content/356/6333/54
