Highly Targeted CRISPR Delivery Advances Gene Editing in Living Animals
Most approved gene therapies today, including those involving CRISPR-Cas9, work their magic on cells removed from the body, after which the edited cells are returned to the patient.
This technique is ideal for targeting blood cells and is currently the method employed in newly approved CRISPR gene therapies for blood diseases like sickle cell anemia, in which edited blood cells are reinfused in patients after their bone marrow has been destroyed by chemotherapy.
A new, precision-targeted delivery method for CRISPR-Cas9, published Jan. 11 in the journal Nature Biotechnology, enables gene editing on very specific subsets of cells while still in the body — a step toward a programmable delivery method that would eliminate the need to obliterate patients' bone marrow and immune system before giving them edited blood cells.
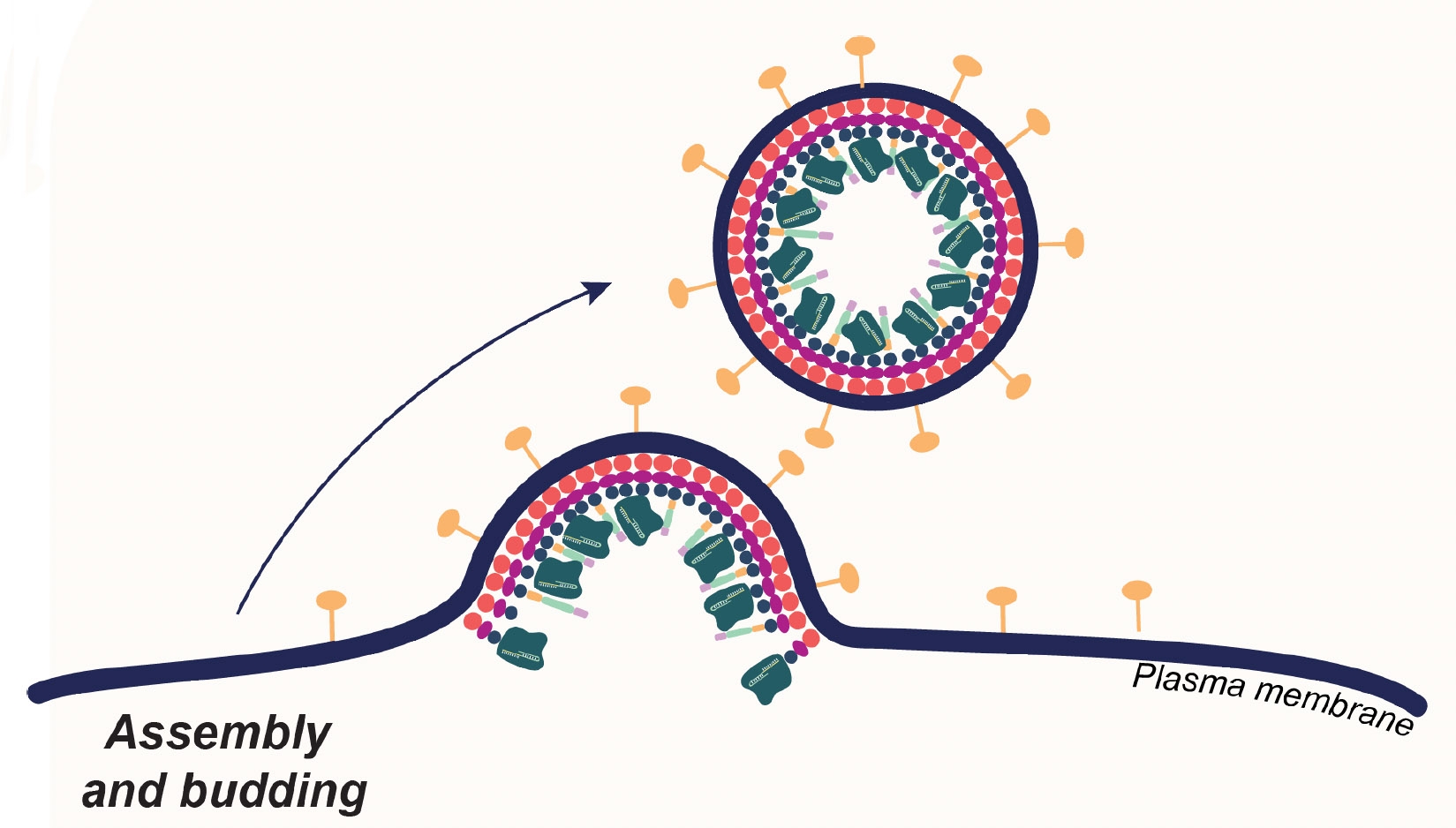
The delivery method, developed in the University of California, Berkeley, laboratory of Jennifer Doudna, co-inventor of CRISPR-Cas9 genome editing, involves wrapping the Cas9 editing proteins and guide RNAs in a membrane bubble that has been decorated with pieces of monoclonal antibodies that home in on specific types of blood cells.
As a demonstration, Jennifer Hamilton, a CRISPR researcher in the Doudna laboratory at the Innovative Genomics Institute (IGI), targeted a cell of the immune system — a T-cell — which is the starting point for a revolutionary cancer treatment called chimeric antigen receptor (CAR) T-cell therapy. Hamilton and her colleagues treated live mice that had been equipped with a humanized immune system and turned their human T-cells into CAR T-cells able to home in on and eliminate another class of immune cell, a B cell.
The feat was a proof of principle, Hamilton said, showing the potential to use this carrier method — enveloped delivery vehicles — to target and edit blood cells and potentially other types of cells in living animals (in vivo) and, eventually, humans.
"Our approach involves multiplexing targeting molecules, that is, having two or more targeting molecules on our particles that interact with their target cell somewhat like an AND gate in a computer," said Hamilton, referring to logic circuits that operate only when two events happen simultaneously. "We were able to get more effective delivery when the particles bound using two antibody ligand interactions. After treating mice with T-cell-targeted vectors, we observed genome engineering in our cell type of interest, T-cells, and not in liver hepatocytes.”
Highly specific targeting is difficult for all methods of delivering genes into cells, she said. Liver cells, in particular, often take up delivery vehicles directed elsewhere.
Viral envelopes
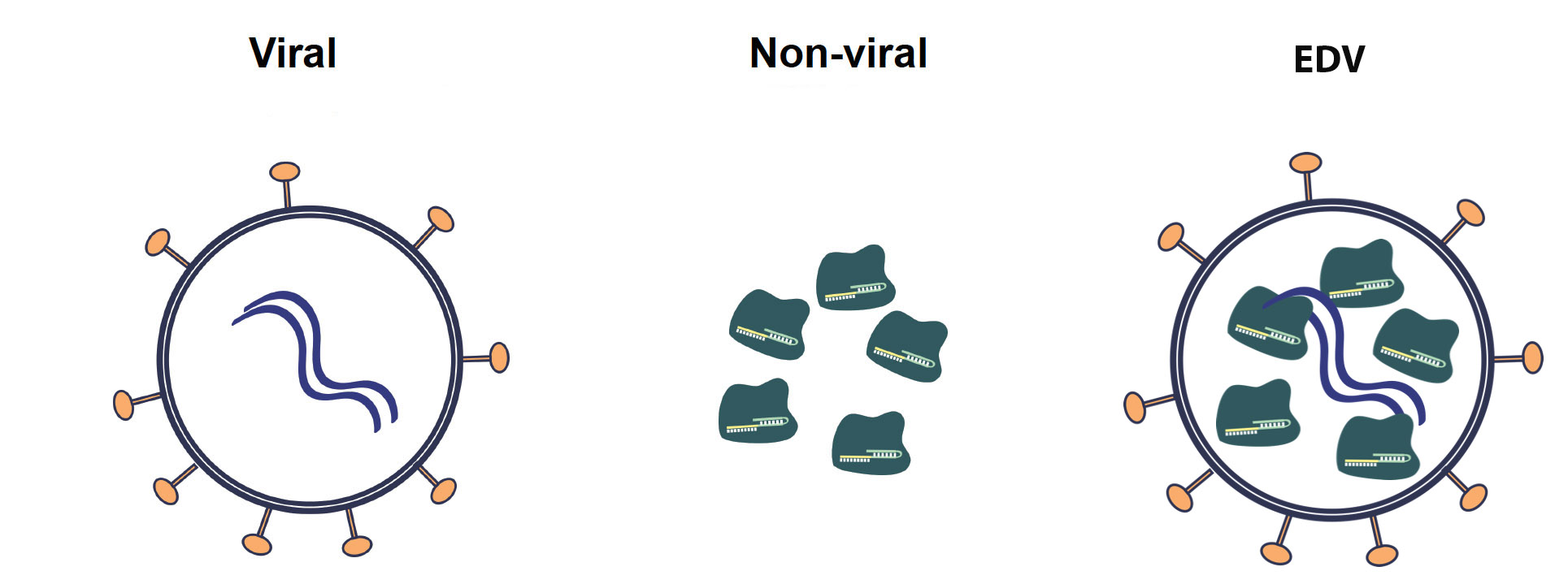
Hamilton and her team are investigating one of several experimental techniques for delivering gene therapies. Many employ the outer coat of encapsulated viruses — the viruses are emptied out and stuffed with corrective transgenes or gene editing tools such as CRISPR-Cas9. Other methods, including one being explored by researchers at IGI, rely on directly injecting cell-penetrating Cas9 proteins into mice to achieve genome editing.
Hamilton, who studied enveloped viruses such as influenza for her Ph.D., focused on engineering that class of viruses because they have a more flexible outer coat, which consists of the exterior membrane of the cell from which they budded.
In a 2021 publication, she demonstrated that the exterior envelope of an HIV-1 virus, which had been gutted and filled with Cas9 and what she called a virus-like particle (VLP), could edit T-cells in culture (ex vivo) and convert them to CAR T-cells. Since then, she has altered the viral envelope so much that she now refers to them as enveloped delivery vehicles, or EDVs.
One key aspect of EDVs is that their outer envelopes can be easily decorated with more than one antibody fragment or targeting ligand, which greatly improves the targeting specificity. Other gene delivery vehicles, such as adeno-associated viruses and lipid nanoparticles, have proven harder to target precisely.
"There are efforts to retarget all of these vectors to have specificity towards one cell type and de-target them against delivery to other cell types," Hamilton said. "You can display antibodies or antibody fragments, like what we've been doing, but the uptake in bystander cells is still quite high. You can bias the delivery into one cell type, but you may still observe uptake in bystander cells. In our paper, we actually looked in the liver to see if we were getting off-target delivery and saw none. I think it would be more challenging to achieve that with a more traditional non-enveloped viral vector or lipid nanoparticle."
In the paper, Hamilton and her colleagues sought to replicate in vivo an ex vivo CRISPR CAR T-cell therapy successfully given to cancer patients that was reported in Science in 2020. That therapy not only delivered a transgene for a receptor targeting cancer cells, but knocked out, using CRISPR, receptors not targeting the cancer.
The UC Berkeley researchers succeeded in knocking out the native T-cell receptor and delivering a transgene for a receptor that targeted B cells — a proxy for cancer cells. Because the Cas9 protein was delivered along with the transgene within the same EDV, it had a shorter life span than methods that deliver a Cas9 gene, which translates to fewer off-target edits.
"What we've tried to achieve in this paper," Hamilton said, "is skipping that whole step of having to engineer cells outside the body. We aimed to systemically administer a single vector that would do both gene delivery and gene knockout in specific cell types inside the body. We used this delivery strategy to make gene-edited CAR T-cells in vivo, in the hopes that we’d be able to streamline the complex process used to manufacture gene-edited CAR T-cells ex vivo."
Doudna and her lab continue to improve the efficiency of EDV-mediated delivery. Hamilton, formerly a postdoctoral researcher in Doudna’s lab, is further developing this delivery method as a fellow in the IGI's Women in Enterprising Science program. The lab's ultimate reason for focusing on vectors that work in vivo is to make CRISPR therapies more broadly available and cheaper. In a recent essay in Wired magazine, Doudna referred to the inequities of today's expensive gene therapies, in part due to extended hospital stays that are required when a patient undergoes a bone marrow transplant.
"The therapy for sickle cell disease is projected to cost over $2 million per patient, and only a small number of facilities in the U.S. have the technological capability to provide it," wrote Doudna, who shared the 2020 Nobel Prize in Chemistry for her co-invention of CRISPR-Cas9 genome editing. "New technologies allowing in vivo delivery of gene-editing therapies and improved manufacturing will be key to driving prices down, as will unique partnerships between universities, government and industry, brought together with affordability as a common goal. It is not enough to simply make the tools. We must ensure they reach those who need them most."
In addition to Hamilton and Doudna, other co-authors of the paper are Evelyn Chen, Barbara Perez, Cindy Sandoval Espinoza, Min Hyung Kang and Marena Trinidad, all affiliated with the IGI and UC Berkeley's Department of Molecular and Cell Biology, and Wayne Ngo of the Gladstone Institutes in San Francisco.
Funding was provided by the National Institutes of Health (RM1HG009490, U01AI142817-02, U19 64542, 64340), U.S. Department of Energy (63645), Emerson Collective and Howard Hughes Medical Institute. Hamilton was supported by the National Institute of General Medical Sciences (K99GM143461-01A1) and the Jane Coffin Childs Memorial Fund for Medical Research.
