Diamonds engage both optical microscopy and MRI for better imaging

When doctors or scientists want to peer into living tissue, there’s always a trade-off between how deep they can probe and how clear a picture they can get.
With light microscopes, researchers can see submicron-resolution structures inside cells or tissue, but only as deep as the millimeter or so that light can penetrate without scattering. Magnetic resonance imaging (MRI) uses radio frequencies that can reach everywhere in the body, but the technique provides low resolution — about a millimeter, or 1,000 times worse than light.
A University of California, Berkeley, researcher has now shown that microscopic diamond tracers can provide information via MRI and optical fluorescence simultaneously, potentially allowing scientists to get high-quality images up to a centimeter below the surface of tissue, 10 times deeper than light alone.
By using two modes of observation, the technique also could allow faster imaging.
The technique would be useful primarily for studying cells and tissue outside the body, probing blood or other fluids for chemical markers of disease, or for physiological studies in animals.
“This is perhaps the first demonstration that the same object can be imaged in optics and hyperpolarized MRI simultaneously,” said Ashok Ajoy, UC Berkeley assistant professor of chemistry. “There is a lot of information you can get in combination, because the two modes are better than the sum of their parts. This opens up many possibilities, where you can accelerate the imaging of these diamond tracers in a medium by several orders of magnitude.”
The technique, which Ajoy and his colleagues report this week in the journal Proceedings of the National Academy of Sciences, utilizes a relatively new type of biological tracer: microdiamonds that have had some of their carbon atoms kicked out and replaced by nitrogen, leaving behind empty spots in the crystal — nitrogen vacancies — that fluoresce when hit by laser light.
Ajoy exploits an isotope of carbon — carbon-13 (C-13) – that occurs naturally in the diamond particles at about 1% concentration, but also could be enriched further by replacing many of the dominant carbon atoms, carbon-12. Carbon-13 nuclei are more readily aligned, or polarized, by nearby spin-polarized vacancy centers, which become polarized at the same time they fluoresce after being illuminated with a laser. The polarized C-13 nuclei yield a stronger signal for nuclear magnetic resonance (NMR) — the technique at the heart of MRI.
As a result, these hyperpolarized diamonds can be detected both optically — because of the fluorescent nitrogen vacancy centers — and at radio frequencies, because of the spin-polarized carbon-13. This allows simultaneous imaging by two of the best techniques available, with particular benefit when looking deep inside tissues that scatter visible light.
“Optical imaging suffers greatly when you go in deep tissue. Even beyond 1 millimeter, you get a lot of optical scattering. This is a major problem,” Ajoy said. “The advantage here is that the imaging can be done in radio frequencies and optical light using the same diamond tracer. The same version of MRI that you use for imaging inside people can be used for imaging these diamond particles, even when the optical fluorescence signature is completely scattered out.”
Detecting nuclear spin
Ajoy focuses on improving NMR — a very precise way of identifying molecules — and its medical imaging counterpart, MRI, in hopes of lowering the cost and reducing the size of the machines. One limitation of NMR and MRI is that large, powerful and costly magnets are needed to align or polarize the nuclear spins of molecules inside samples or the body so that they can be detected by pulses of radio waves. But humans can’t withstand the very high magnetic fields needed to get lots of spins polarized at once, which would provide better images.

One way to overcome this is to tweak the nuclear spins of the atoms you want to detect so that more of them are aligned in the same direction, instead of randomly. With more spins aligned, called hyperpolarization, the signal detected by radio is stronger, and less powerful magnets can be used.
In his latest experiments, Ajoy employed a magnetic field equivalent to that of a cheap refrigerator magnet and an inexpensive green laser to hyperpolarize the carbon-13 atoms in the crystal lattice of the microdiamonds.
“It turns out that if you shine light on these particles, you can align their spins to a very, very high degree — about three to four orders of magnitude higher than the alignment of spins in an MRI machine,” Ajoy said. “Compared to conventional hospital MRIs, which use a magnetic field of 1.5 teslas, the carbons are polarized effectively like they were in a 1,000-tesla magnetic field.”
When the diamonds are targeted to specific sites in cells or tissue — by antibodies, for example, which are often used with fluorescent tracers — they can be detected both by NMR imaging of the hyperpolarized C-13 and the fluorescence of the nitrogen vacancy centers in the diamond. The nitrogen vacancy-center diamonds are already becoming more widely used as tracers for their fluorescence alone.
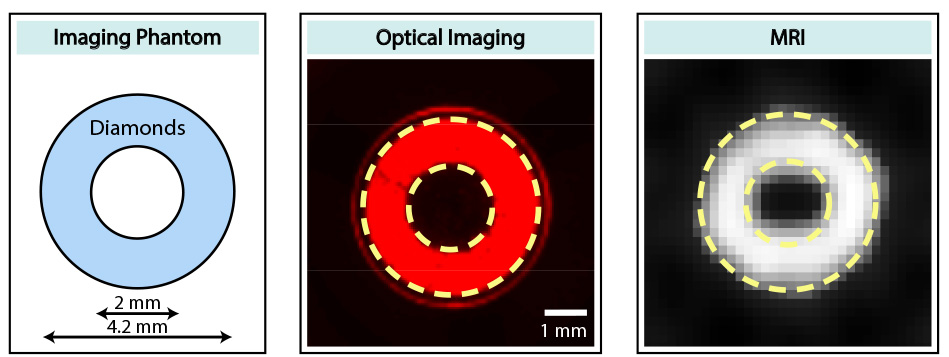
“We show one important cool feature of these diamond particles, the fact that they spin polarize — therefore they can glow very bright in an MRI machine — but they also fluoresce optically,” he said. “The same thing that endows them with the spin polarization also allows them to fluoresce optically.”
The diamond tracers also are inexpensive and relatively easy to work with, Ajoy said. Together, these new developments could, in the future, allow for an inexpensive NMR imaging machine on every chemist’s benchtop. Today, only large hospitals can afford the million-dollar price tag for MRIs. He currently is working on other techniques to improve NMR and MRI, including using hyperpolarized diamond particles to hyperpolarize other molecules.
The experiments were led by former graduate student Xudong Lv using a home-built hyperpolarizer device constructed by staff scientist Emanuel Druga. Ajoy’s work was supported by the Office of Naval Research (N00014-20-1-2806). Other co-authors are F. Wang, A. Aguilar, T. McKnelly, R. Nazaryan and L. Wu of UC Berkeley; J. H. Walton of UC Davis; O. Shenderova of Adamas Nanotechnologies Inc., in North Carolina; D. B. Vigneron of UCSF; Carlos Meriles of the City University of New York; and professor of chemical and biomolecular engineering Jeffrey Reimer and chemistry professor Alexander Pines, both of UC Berkeley.
RELATED INFORMATION
