Breaking Kasha’s Rule
Observation of a scientific rule being broken can sometimes lead to new knowledge and important applications. Such would seem to be the case when scientists with the U.S. Department of Energy (DOE)’s Lawrence Berkeley National Laboratory (Berkeley Lab) created artificial molecules of semiconductor nanocrystals and watched them break a fundamental principle of photoluminescence known as “Kasha’s rule.”
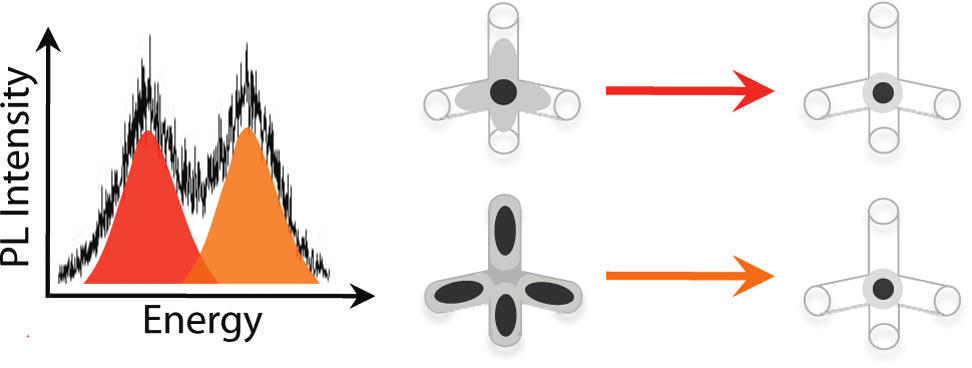
Named for chemist Michael Kasha, who proposed it in 1950, Kasha’s rule holds that when light is shined on a molecule, the molecule will only emit light (fluorescence or phosphorescence) from its lowest energy excited state. While there have been examples of organic molecules, such as azulene, that break Kasha’s rule, these examples are rare. Highly luminescent molecular systems crafted from quantum dots that break Kasha’s rule have not been reported – until now.
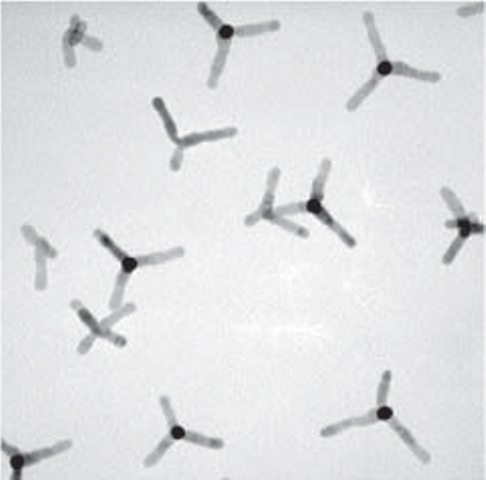
”We have demonstrated a semiconductor nanocrystal molecule, in the form of a tetrapod consisting of a cadmium-selenide quantum dot core and four cadmium sulfide arms, that breaks Kasha’s rule by emitting light from multiple excited states,” says Paul Alivisatos, director of Berkeley Lab and the Larry and Diane Bock Professor of Nanotechnology at the University of California (UC) Berkeley. “Because this nanocrystal system has much higher quantum yield and is relatively more photostable than organic molecules, it holds promising potential for optical sensing and light emission-based applications, such as LEDs and imaging labels.”
Alivisatos, an internationally recognized authority on nanochemistry, is one of two corresponding authors, along with Sanjeevi Sivasankar of DOE’s Ames Laboratory and Iowa State University, on a paper describing this work in the journal Nano Letters. The paper is titled “Spatially Indirect Emission in a Luminescent Nanocrystal Molecule.” Co-authoring the paper were Charina Choi, Prashant Jain and Andrew Olson, all members of Alivisatos’ research group, plus Hui Li, a member of Sivasankar’s research group.
Semiconductor tetrapods make exceptionally good subjects for the study of electronically coupled nanocrystals as Charina Choi, lead author of the Nano Letters paper, explains.
“For the study of nanocrystal molecules, it is important to be able to grow complex nanocrystals in which simple nanocrystal building blocks are connected together in well-defined ways,” Choi says. “Although there are many versions of electronically coupled nanocrystal molecules, semiconductor tetrapods feature a beautiful symmetry that is analogous to the methane molecule, one of the fundamental units of organic chemistry.”
In this study, Choi, Alivisatos and their co-authors designed a cadmium-selenide (CdSe)and cadmium-sulfide (CdS) core/shell tetrapod whose quasi-type-I band alignment results in high luminescence quantum yields of 30- to 60-percent. The highest occupied molecular orbital (HOMO) of this tetrapod involves an electron “hole” within the CdSe core, while the lowest unoccupied molecular orbital (LUMO) is centered within the core but is also likely to be present in the four arms as well. The next lowest unoccupied molecular orbital (LUMO+1) is located primarily within the four CdS arms.
Through single particle photoluminescence spectroscopy carried out at Ames, it was determined that when a CdSe/CdS core/shell tetrapod is excited, not only is a photon emitted at the HOMO-LUMO energy gap as expected, but there is also a second photon emitted at a higher energy that corresponds to a transition to the HOMO from the LUMO+1.
“The discovery that these CdSe/CdS core/shell tetrapods emit two colors was a surprise,” Choi says. “If we can learn to control the frequency and intensity of the emitted colors then these tetrapods may be useful for multi-color emission technologies.”
For example, says co-author Prashant Jain, “In the field of optical sensing with light emitters, it is impractical to rely simply on changes in emission intensity as emission intensity can fluctuate significantly due to background signal. However, if a molecule emits light from multiple excited states, then one can design a ratiometric sensor, which would provide more accurate readouts than intensity magnitude, and would be more robust against fluctuations and background signals.”
Another promising possibility for CdSe/CdS core/shell tetrapods is their potential application as nanoscale sensors for measuring forces. Previous work by Alivisatos and Choi showed that the emission wavelengths of these tetrapods will shift in response to local stress on their four arms.
“When a stress bends the arms of a tetrapod it perturbs the electronic coupling within the tetrapod’s heterostructure, which in turn changes the color of the emitted light, and also likely alters the ratio of emission intensity from the two excited states,” Choi says. “We are currently trying to use this dependence to measure biological forces, for example, the stresses exerted by a beating heart cell.”
By adjusting the length of a CdSe/CdS core/shell tetrapod’s arms, it is possible to tune band alignment and electronic coupling within the heterostructure. The result would be tunable emissions from multiple excited states, an important advantage for nano-optic applications.
“We’ve demonstrated that the oscillator strength of LUMO+1 to HOMO light emissions can be tuned by changing the arm length of the tetrapod,” Choi says. “We predict that the lifetime and energy of the emissions can also be controlled through appropriate structural modifications, including arm thickness, number of arms, chemical composition and particle strain.”
This research was primarily supported by DOE’s Office of Science.
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 12 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
Additional Information
For more information about the research of Paul Alivisatos, visit the Website at http://www.cchem.berkeley.edu/pagrp/
For more information about the research of Prashant Jain, visit the Website at http://www.nanogold.org/
For more information about the research of Sanjeevi Sivasankar visit his Website at http://sivasankar.physics.iastate.edu/
For more information about the Ames Laboratory visit the Website at http://www.ameslab.gov/home
