Research Expertise and Interest
eukaryotic molecular biology; biochemistry, cellular differentiation, chromatin function, RNA synthesis, single cell imaging, single molecule imaging
Research Description
Robert Tjian is Professor of Biochemistry, Biophysics and Structural Biology, the Li Ka Shing Chancellor's Chair in Biology, and a HHMI Investigator.
Biochemistry of Transcription and Chromatin Transactions: Over the past 30 years, the Tjian + Darzacq Group lab has identified, isolated and characterized a large number (~100) of essential drosophila and human transcription factors. These regulatory proteins include enhancer/promoter recognition factors, core RNA pol II initiation factors and co-activators that form large multi-subunit complexes at promoter DNA to mediate transcription initiation and decode the genome. Their studies indicate that large co-activator complexes play a critical role in mediating both universal as well as cell type specific networks of gene transcription and can serve as the interface between transcription and chromatin regulation. Although their earlier studies focused on in vitro biochemistry and conventional molecular genetics approaches, their recent research is directed at the more challenging task of integrating specific molecular transactions with in vivo mechanisms in cells and organisms under physiological conditions.
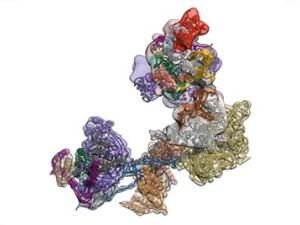
Single Cell Biochemistry, Structural Biology and Single Molecule Measurements: In order to address mechanisms of transcriptional control they use a combination of structural biological methods such as cryo EM/3D particle reconstruction, X-ray crystallography and single molecule measurements. Another effort is to develop strategies for measuring the assembly and movement of transcription complexes at the single cell level. These studies aim to track the movement and function of target transcription factors in live cells by various optical methods. In addition, they continue to utilize tools such as siRNA mediated knockdown of transcription factors as well as in vitro reconstitution of regulated transcription to link specific molecular mechanisms of control with in vivo physiology.
Transcription Mechanisms in Stem Cell Pluripotency and Differentiation: Deciphering the molecular basis of pluripotency is fundamental to our understanding of development and embryonic stem (ES) cell function. Using techniques such as ChIP-sequence analysis, systematic RNAi mutagenesis, transgenic animals or cells, and in vitro transcription assays, they have developed complimentary experimental strategies to dissect in vivo mechanisms of transcriptional regulation and tease out how these various protein/DNA and protein/protein transactions influence gene expression. They recently found that TAF3, a TBP-associated core promoter factor, is highly enriched in ES cells, where it is required for endoderm lineage differentiation and prevents premature specification of neuroectoderm and mesoderm. In addition to its role in the core promoter recognition complex TFIID, genome-wide binding studies reveal that TAF3 localizes to chromosomal regions bound by CTCF and cohesin. Enrichment for TAF3/CTCF/cohesin bound regions distinguishes TAF3-activated from TAF3-repressed genes. Notably, CTCF directly interacts with and recruits TAF3 to promoter distal sites and TAF3-dependent DNA looping is observed between the promoter distal sites and core promoters occupied by TAF3/CTCF/cohesin. Their findings thus support a new role of TAF3 in mediating long-range chromatin regulatory interactions to safeguard the finely balanced transcriptional programs that give rise to pluripotency.
In a related set of experiments, They have studied how the transcriptional activators Oct4, Sox2 and Nanog cooperate with a wide array of cofactors to orchestrate an ES cell-specific gene expression program that forms the molecular basis of pluripotency. They have used an unbiased in vitro transcription-biochemical complementation assay to discover a multi-subunit stem cell coactivator complex (SCC) that is selectively required for the synergistic activation of the Nanog gene by Oct4 and Sox2. Purification, identification and reconstitution of SCC revealed this coactivator to be the trimeric XPC-nucleotide excision repair complex. SCC interacts directly with Oct4 and Sox2 and is recruited to the Nanog and Oct4 promoters as well as a majority of genomic regions that are occupied by Oct4 and Sox2. Depletion of SCC/XPC compromised both pluripotency in ES cells and somatic cell reprogramming of fibroblasts to induced pluripotent stem cells, illustrating its diversified functions in maintaining ES cell pluripotency and safeguarding genome integrity.
In the News
New Fellows of the American Association for the Advancement of Science
Freeze-frame microscopy captures molecule’s ‘lock-and-load’ on DNA
Scientists decipher opening dance steps of DNA and its partner
Working at temperatures near absolute zero, UC Berkeley experts in electron microscopy have learned in detail how proteins orchestrate the first key steps in gene activation – opening up the double-stranded DNA.