On Memory’s Trail
Memories shape our lives. They keep us connected to who we love, what we value and what we know. And they allow us to find our way home.
Yet how the brain makes, stores and retrieves memories remains largely a mystery. Many neuroscientists think that by tracking the brain’s neurons as they make connections and communicate, we can begin to understand the normal process of memory and the missteps that steal memory away.
“It’s clear that Alzheimer’s kills certain kinds of cells, but how this occurs and how it might be prevented is unknown,” says Berkeley neuroscientist Ehud (Udi) Isacoff, professor of molecular and cell biology and director of Berkeley’s Helen Wills Neuroscience Institute. “Is it sufficient to restore those cells, or is there a way to restore memory without actually needing to re-activate the cells that have died? If you don’t know what memory is, how can you answer those kinds of questions?”
Isacoff and his colleagues explore the brain at several levels critical to ultimately understand how memories form and what can threaten their demise. They study how molecular interactions drive neuron-to-neuron communication and influence how neurons “wire up” to form networks during embryonic development.
Their research also examines at both the molecular and cellular level how the timing and pattern of neuron activity in the central nervous system advances from an apparently random state to a tightly choreographed performance that underlies the dart of a fish or the coordinated walk of bipeds like us.
And recently, Isacoff’s research has extended to experiments that trace real-time memory formation in living animals.
The switch that starts electrical signaling between the brain’s neurons is found, of course, at the synapse. Here, neurotransmitter proteins convert a chemical signal from one neuron into an electrical signal that races down the conducting cable of a neighboring neuron at breakneck speed.
Isacoff’s lab teases apart the ways in which neurotransmitters and their receptors across the synapse generate the electrical impulses that allow communication in the brain.
Key studies in the lab have exploited fluorescent sensor proteins that change brightness when neurons are active. The sensors have helped reveal the startlingly brief window of time during which spinal cord neurons “get it together” to enable larval fish to swim.
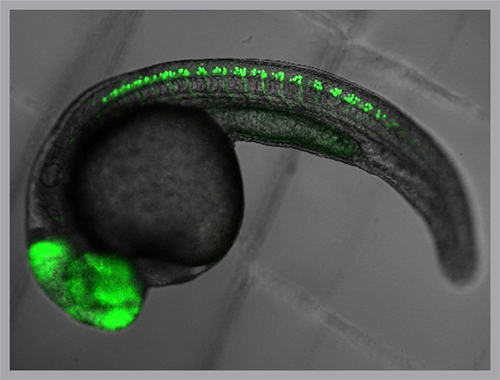
The research found that in just two hours on the first day of larval development, the random pattern of neurons firing — like fireflies flashing on a summer night — changes to a tight Rockettes-like synchronicity. The transition to this highly regimented neuron-to-neuron communication enables coordinated tail movement.
“The speed at which order emerges from chaos in this circuit is astonishing,” Isacoff says.
A very different tool, developed by the lab, is now allowing his group and others to manipulate neuronal activity in living animals to track circuit formation and identify the adjustments in connections that underlie normal function and learning.
Over the past 11 years, Isacoff and two close colleagues, Berkeley biologist Richard Kramer and chemist Dirk Trauner at Ludwig-Maximilians University (LMU) Munich, developed techniques that harness light to block or mimic the function of neuronal synapses. The research focuses on the neurotransmitter glutamate and its receptor across the synapse.
The key player in the research is a protein commonly used in food coloring that, surprisingly, changes shape when exposed to light. The scientists link the molecule to glutamate and its receptor on individual neurons. When light is beamed at the neuron, the protein changes shape, which brings the two together — just as if the cell had received glutamate from a neuron across the synapse.
The misled neuron then boosts its signal, increasing information flow through its circuit. A complementary approach can be used to block such communication.
Isacoff, Kramer and Trauner, along with Helen Wills Neuroscience Institute faculty scientists John Flannery, David Schaffer and Yang Dan have already used the novel strategy to develop a potential cure for a form of blindness that affects about 100,000 people in the U.S.
The condition, known as retinitis pigmentosa, can lead to blindness when a single layer of photoreceptor cells in the retina die. In research with mice, the photoswitch-tricked glutamate receptors allowed surviving cell layers to restore the retina’s ability to detect light, and so restore vision. This strategy may provide a cure for the disease in humans.
Much more challenging than making a chemical-biological prosthetic device is Isacoff’s new effort to understand how individual neurons and groups of neurons coordinate complex behavior to form and access memory. His lab is starting to use the photoswitch technology to turn on and off neuron signals as animals perform normal behaviors and learn new tasks.
A petite freshwater minnow, the zebrafish, has become a favorite model for studying genetics, disease and behavior. Its genome has been mapped; its brain, though small, is complex enough to coordinate a wide range of behavior.
But the fish possesses an extraordinary trait that makes Isacoff’s neurological research possible: during its larval stage, a zebrafish is utterly transparent. Every cell in its body, including each of the 100,000 neurons in its brain, can be reached by light without interfering with natural development or behavior.
In this way, light can be used to control whether, when and how a neuron signals to its neighbors — an essential step for both the development of neural circuits and for learning and memory.
“Imagine this: 100,000 neurons in a brain that performs many operations just like our own brain does,” Isacoff says. “But in zebrafish we can watch the activity of the entire lot during behavior.
“Then, we can project patterns of light onto the brain to play back this activity or to adjust the strength of communication between neurons and see how behavior and learning change. Finally, the tools are in place to decipher how brain circuits change to develop new capacities and maybe even how they remember.”
Related information
