How To Grow Back The Back - Engineered Cartilage Surfaces
You’re going to shrink today. You did yesterday, and you will again tomorrow. By bedtime every night, you’re likely to be about an inch shorter than when you got up. But assuming you sleep lying down, each evening’s rest restores you to full height.

The spongy discs between each vertebra lose fluid from the forces that bear down on them during the day. They become thinner and stiffer. In a healthy back, discs reabsorb the fluid when the body goes horizontal for the night.
Herniated discs don’t bounce back. When disc tissue squeezes out of the space, they lose some of their shock absorbing capacity and impinge on spinal nerves, triggering lower back and leg pain. Herniated discs and severe disc degeneration inevitably change the biomechanical forces on tissues and structures in and near the back.
But how damaged discs alter the body’s biomechanics remains largely unknown. Even the mechanical stresses caused by treating herniated disc disease is poorly understood.
Understanding the biomechanics of healthy and damaged discs could lead to new
treatments that reduce physical stress. Insights might reduce the need to cut out disc material or fuse vertebrae in many patients. Farther down the line, but already under study at UC Berkeley, new disc tissue could be grown – engineered in the lab to replace deteriorated discs. Some 20 years of research has taken this futuristic-sounding prospect into the first clinical trials for repairing or replacing damaged cartilage.
UC Berkeley’s Grace O’Connell has been at the heart of this research since graduate school. Now in her second year as assistant professor of mechanical engineering, she is adapting the technology with the aim of growing new human disc tissue.
Source material comes from donated disc tissue excised during back surgeries at UC Davis Medical Center, posing an initial problem. “The tissue comes from an already-compromised environment – serious disc damage that required surgery,” she says. “Starting with unhealthy disc tissue requires more culture time in the lab to ‘reprogram’ cells to produce new tissues.”
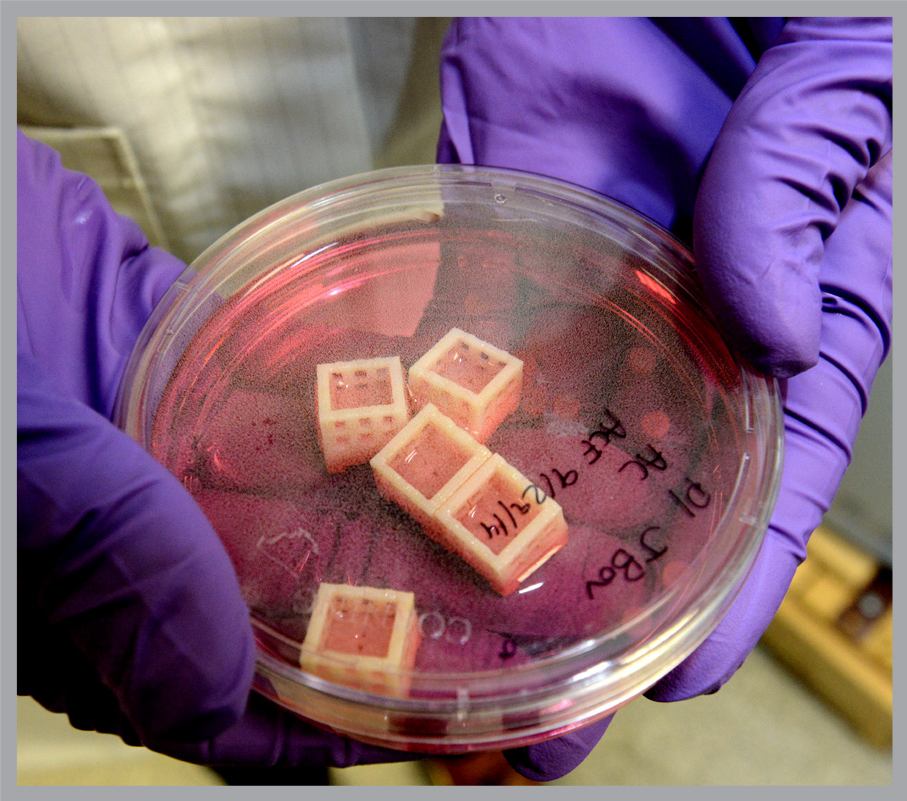
The lab made initial progress with an animal model – disc material from cow tails supplied by butchers. Refining key steps in this process will likely help make the transition from animal to human disc tissue engineering.
How do you grow new, living tissue in the lab? First the bovine disc material is digested with an enzyme and the disc cells are separated out. They are mixed into a soft, transparent algae based-gel, meant to mimic the spongy environment of normal discs and provide a “scaffold” on which the new cells can grow.
The gel is key. O’Connell’s postdoc research focused on developing a gel scaffold for growing cartilage. Though cartilage is similar to disc tissue, it is stiffer and does not swell during bed rest like disc tissue. O’Connell’s lab uses softer, more pliable gel and culture conditions designed to increase swelling capabilities of engineered disc tissues.
The cells are supplied with nutrients the body normally provides during development. O’Connell casts the gel containing the cells into sheets, and then uses a “biopsy punch” to create four-millimeter-diameter cylinders of gel and cells in which the cells grow new tissue. After four to six weeks, each cylinder contains about 800,000 cultured cells embedded in and supported by the gel, with properties comparable to native tissue.
Clinical application of this technology will require much larger engineered tissues, O’Connell says. Berkeley’s Rose Hill Innovator Award is providing funding for three years to help her scale up the pioneering effort and to develop tissues that better mimic the anatomy of the human joint. O’Connell hopes that “in the next three years we will be able to demonstrate that this technology can be used as a biological repair strategy for herniated discs, and gain NIH support to advance it.”
O’Connell directs Berkeley’s Soft Tissue Biomechanics Lab in the Department of Mechanical Engineering. The lab measures how normal discs perform under the loads placed on them by daily activity. “We want to understand the mechanical function of these tissues and how they change with age, degeneration and injury,” O’Connell says. “ It’s important to know how the healthy tissue behaves mechanically if we are going to design better repair strategies that mimic this function.”
The lab recently demonstrated how compression of bovine discs for four hours squeezes fluid out of it, making it thinner and stiffer – comparable to the effect of a day’s normal activity for humans. The study showed that the disc regained fluid and was restored after the equivalent of a night’s reduction of pressure. The research may help provide a better picture of how daily activity makes discs more susceptible to tissue damage.
O’Connell advises two graduate students, but her research group also includes seven undergrads. “So many undergrads are looking for research experience. That’s something I want to support. In undergraduate class and lab sessions, students learn the technical skills needed to be a successful engineer. But the hands-on experience in the lab allows students to learn trouble shooting and actual implementation from an idea to a solution. That’s what’s needed to keep research moving forward.”
_________________________________
The Rose Hills Innovator Program supports distinguished early-career faculty at UC Berkeley interested in developing highly innovative research programs in the fields of science, technology, engineering and math (STEM). The program intends to strengthen the efforts of UC Berkeley’s world-class faculty by providing seed support for projects with exceptionally high scientific promise. For more information, see rosehillsinnovators.berkeley.edu.
Additional Information
Research profile on Grace O'Connell
