Research Bio
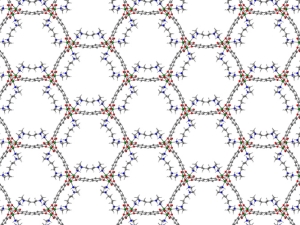
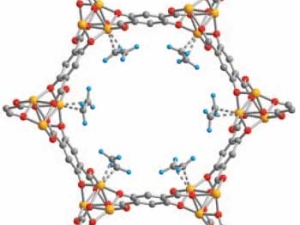
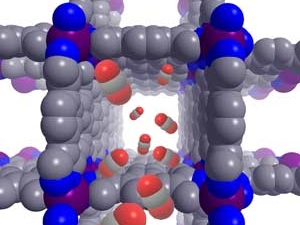
Jeffrey R. Long is an inorganic chemist who designs new materials for energy and environmental applications. He is best known for pioneering work on metal–organic frameworks (MOFs) and porous polymers for gas storage, separations, catalysis, and recovery of critical metal ions. He further leads an effort to use molecular design principles to create new magnetic molecules and materials. His research has developed novel approaches to capturing carbon dioxide, storing hydrogen, removing toxic and high-value metal-ions from aqueous sources, and generating highly coercive magnets.
Long is Professor of Chemistry, Chemical and Biomolecular Engineering, and Materials Science and Engineering and Director of the Baker Hughes Institute for Decarbonization Materials at UC Berkeley, a Senior Faculty Scientist at Lawrence Berkeley National Laboratory, a Fellow of the American Academy of Arts and Sciences, and a member of the National Academy of Science. His work has been recognized with the Eni Award Energy Transition Prize, the Royal Society of Chemistry Ludwig Mond Award, and the American Chemical Society F. Albert Cotton Award in Synthetic Inorganic Chemistry.
Research Expertise and Interest
inorganic and materials chemistry, synthesis of inorganic molecules and higher dimensional solids, precise tailoring of chemical and physical properties, gas storage, molecular separations and catalysis in porous materials, magnetic and conductive materials
In the News
Capturing Wellhead Gases for Profit and a Cleaner Environment
National Academy of Sciences Elects Seven From UC Berkeley
A Big Step Toward ‘Green’ Ammonia and a ‘Greener’ Fertilizer
Improved desalination process also removes toxic metals to produce clean water
New technique to capture CO2 could reduce power plant greenhouse gases
A Chain Reaction to Spare the Air
New Material Captures Carbon at Half the Energy Cost
Capturing carbon from power plants is likely in the future to avoid the worst effects of climate change, but current technologies are very expensive. A new material, a diamine-appended metal-organic framework, captures and releases CO2 with much reduced energy costs compared to today’s technologies, potentially lowering the cost of capturing this greenhouse gas.
New material cuts energy costs of separating gas for plastics and fuels
A new type of hybrid material developed at UC Berkeley could help oil and chemical companies save energy and money by eliminating an energy-intensive gas-separation process.
Breakthrough in designing cheaper, more efficient catalysts for fuel cells
UC Berkeley chemists Chris Chang, Jeff Long and Marcin Majda have redesigned catalysts in ways that could have a profound impact on the chemical industry as well as on the growing market for hydrogen fuel cell vehicles.
Berkeley Lab to Develop Novel Materials for Hydrogen Storage
Lawrence Berkeley National Laboratory is aiming to solve how to store enough of hydrogen-powered fuel cells, in a safe and cost-effective manner, to power a vehicle for 300 miles by synthesizing novel materials with high hydrogen adsorption capacities.
Capturing carbon
Researchers at Berkeley and other universities to find ways to capture carbon dioxide, produced by burning coal and natural gas, from the waste stream of power plants so that it can be sequestered underground.
Berkeley Lab to Receive $8.6 Million in Recovery Act Funding for "Transformational" Energy Research Projects
The U.S. Department of Energy (DOE)'s Lawrence Berkeley National Laboratory (Berkeley Lab) has been awarded $8.6 million in Recovery Act funding for what the DOE calls "ambitious research projects that could fundamentally change the way the country uses and produces energy."
$30 million from DOE for carbon capture, sequestration
Two UC Berkeley faculty members will receive $30 million over the next five years from the U.S. Department of Energy to find better ways to separate carbon dioxide from power plant and natural gas well emissions and stick it permanently underground.