Research Expertise and Interest
stem cell niche engineering, tissue repair, stem cell aging and rejuvenation
Research Description
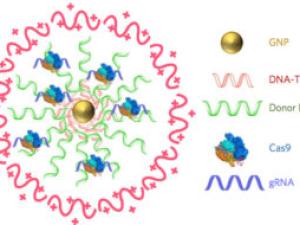
Irina Conboy is a professor in the Department of Bioengineering. A key direction of research in her lab is to understand age-imposed and pathological changes in molecular compositions of systemic and local environments of adult stem cells and to calibrate these to health - youth. In the past few years this direction has been ramified into synthetic biology, CRISPR technologies, bio-orhtogonal proteomics and development of innovative digital bio-sensors that have been collaboratively applied to the fields of aging and diagnostics of genetic diseases. Resolving the conundrum of whether aging is a disease and what biomarks it, they posited that both, aging and disease, are biomarked not by the levels of gene expression or proteins, but their noise; and they identified the noise detectors (UC Berkeley IP) that reveal the natural polynomial curve of aging and allow quantifying (not predicting) the biological age. Success in their research will improve our understanding of the determinants of homeostatic health and will enable novel rational approaches to treat a number of degenerative, fibrotic, metabolic and inflammatory diseases, that often accompany human aging, as a class.
Links that cover and prioritize research in aging:
- https://www.aging-us.com/issue/v15i17#cover-6504862fb01de70008c981c8, https://doi.org/10.18632/aging.205046
- https://engineering.berkeley.edu/news/2023/05/forever-young/
- https://engineering.berkeley.edu/news/2020/11/young-again/
- https://engineering.berkeley.edu/news/2019/10/new-frontiers-in-gene-editing/
- https://www.economist.com/science-and-technology/2017/07/15/blood-from-young-animals-can-revitalise-old-ones
- https://www.sciencedaily.com/releases/2016/11/161122123102.htm
- https://time.com/4579899/young-blood-transfusion-aging/
- https://www.kqed.org/science/19381/new-uc-berkeley-study-shows-oxytocin-may-help-rejuvenate-aging-muscles
In the News
Study Finds Medical Procedure That Rejuvenates Old Human Blood
CRISPR-Chip advance streamlines genetic testing for medical diagnostics and research
Diluting blood plasma rejuvenates tissue, reverses aging in mice
New CRISPR-powered device detects genetic mutations in minutes
CRISPR-Gold fixes Duchenne muscular dystrophy mutation in mice
Viral infections decrease muscle health, cause other collateral damage
Drug Perks Up Old Muscles and Aging Brains
UC Berkeley researchers have discovered that a small-molecule drug simultaneously perks up old stem cells in the brains and muscles of mice, a finding that could lead to drug interventions for humans that would make aging tissues throughout the body act young again.
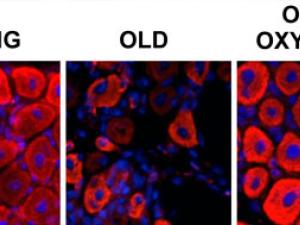
‘Trust hormone’ oxytocin helps old muscle work like new, study finds
UC Berkeley researchers have discovered that oxytocin – a hormone associated with maternal nurturing, social attachments, childbirth and sex – is indispensable for healthy muscle maintenance and repair, and that in mice it declines with age.
Bioengineers reprogram muscles to combat degeneration
UC Berkeley researchers have turned back the clock on mature muscle tissue, coaxing it back to an earlier stem cell stage to form new muscle. Moreover, they showed in mice that the newly reprogrammed muscle stem cells could be used to help repair damaged tissue. The achievement is described in the Sept. 23 issue of the journal Chemistry & Biology.