
Shawn Shadden
Mechanical EngineeringShawn Shadden is Assistant Professor in the Department of Mechanical Engineering. His research focuses on the advancement of theoretical and computational methods to quantify complex fluid flow. Shawn received his PhD in Control and Dynamical Systems from the California Institute of Technology and his BS from the University of Texas, Austin in Aerospace Engineering.
Spark Award Project
Image-Based Modeling for Acute Stroke Diagnosis
Diagnostic imaging, including CT and MRI, has transformed medicine by enabling noninvasive view of tissue inside the body. Using this technology to reliably diagnose cardiovascular problems is hindered by the fact that functional information about blood flow can be difficult to glean from medical images. Moreover, while diagnostic imaging may be useful to spot a problem, it does not provide the predictive power needed to test whether a given intervention may have anticipated benefit. The integration of diagnostic imaging with computational modeling can help address these two critical needs. In regards to ischemic stroke, we aim to develop a quantitative metric derived from image-based blood flow modeling at the time of diagnosis to risk-stratify the patient for a given treatment. The physician can then use this information, along with the patient’s clinical data, to make a more informed and accurate treatment decision for that particular patient.
Shawn Shadden’s Story
Rushed to the hospital with a crushing headache and numbness on his left side, the 72-year-old man appears to be having a stroke — either a bleed or a block in a major artery supplying the brain.

A CT scan shows a blockage, but it’s hard to gauge how much the blockage hinders blood flow. Should the patient receive injections of a tissue plasminogen activator to try to dissolve the clot, or should he undergo a more invasive procedure to save his brain from oxygen starvation?
There are so many unknowns: Do blood vessel constrictions that show up on the CT scan threaten increased danger, or are they innocuous? Will this patient’s arterial system likely find a way to reroute the blood flow?
The configuration and capacity of the brain’s vascular system vary widely from one person to another. Even when the scan shows an unusual number of tight turns and ominous narrowings in the blood vessel network, the vasculature can often safely reroute the blood flow from a blockage and protect the brain.
A drop in blood pressure at the blockage site and at other points along the blood vessel network would signal the degree of danger. But imaging can’t provide this level of physiological detail.
Measurements of blood flow and pressure could greatly boost the ability to predict outcomes in real time — to decide if invasive treatment is urgently needed, or if it can be ruled out.
Research in the past ten years shows that the information in diagnostic imaging can be converted through intense computational strategies into a highly detailed model of blood flow and pressure in regions of the heart, arteries or veins. The technology can pinpoint telltale changes at different sites — a potentially life-saving look at the state of blood flow in particular regions of the body.
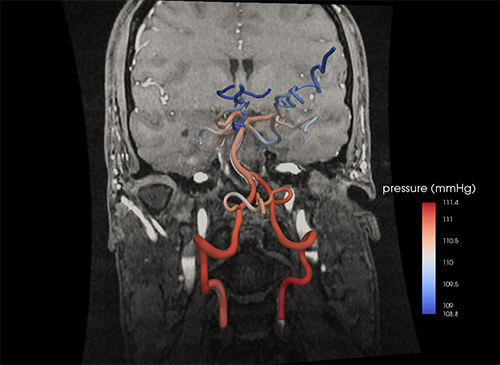
“We want to know how much blood flow is going down each blood vessel at every point along the varying geometry in a patient’s heart during coronary artery disease, or brain during a cerebrovascular accident,” says Shawn Shadden, assistant professor of mechanical engineering at UC Berkeley.
As a postdoc, Shadden helped develop computational strategies to evaluate and characterize blood flow under various disease conditions. He is a consultant for a start-up company that is using the modeling to predict which patients suffering from coronary artery disease need a stent to restore adequate heart performance.
In his lab on the north side of the Berkeley campus, he now applies this kind of computational approach to model blood flow and pressure in the brain during a stroke.
Starting with a CT or MR scan, he develops a computer model that will allow him to simulate the millimeter-by-millimeter performance of a person’s brain vasculature.
The model mathematically breaks down the 3-D space inside a blood vessel into separate volumes along its entire route, and solves for blood flow velocity and pressure at each point.
The calculations can provide a personalized view of brain vasculature. It separates the real risks from apparent ones in the arteries’ twists and turns, the flow of blood past minor congenital constrictions or plaque-buildups.
“It is difficult to obtain this data from the diagnostic imaging, but imaging does provide us with a patient’s unique vascular anatomy, and from this we can solve for the blood flow and pressure in each area. That allows us to predict how it might change if an intervention is performed,” Shadden says.
His stroke assessment research is supported by a five-year Bakar Fellowship to demonstrate the clinical promise of this model-based approach and to provide mentoring to help get from the lab into the commercial world.
“Science is one thing, but when you talk about making clinical advances, there are so many steps you need to take,” Shadden says. “Besides working with regulatory agencies, you need to know how to identify and protect the core intellectual property, and connect with the investors and other scientists who have created start-ups. “We’re just at the early stages of the Bakar support, but it’s designed to help us move through these steps that are new to me.”
He has been collaborating with his Berkeley colleague Tony Keaveny who is already bringing to market a computer model of bone fracture risk from osteoporosis. Using existing clinical stroke data, Shadden and Keaveny plan first to carry out retrospective studies, comparing a stroke patient’s actual outcome with that predicted by the blood circulation model. From there, the goal would be prospective studies to aid clinicians in real-time assessments.
“I think of it as almost like a blood test,” Shadden says. “The lab reports a number, and the measure is high or low, and that helps guide the treatment decision.”
If it works as intended, the blood pressure model should help customize stroke treatment, limit aggressive interventions and in the process reduce risk or unnecessary costs. Pretty good for a lab test.