
Jay Keasling
Chemical and Biomolecular EngineeringJay Keasling is a Professor in the Department of Chemical & Biomolecular Engineering and Bioengineering. He is also Senior Faculty Scientist at the Lawrence Berkeley National Laboratory, and his research interests include metabolic engineering of microorganisms.
Spark Award Project
Skin probiotics to treat disease
Aging and disease cause damaged, inflamed skin. Multiple factors can cause and aggravate the skin, including protein imbalances, inflammatory cytokines, reactive oxygen species, and harmful bacteria. Conventional topical creams often fail to address these conditions due to the limited lifetime of their active ingredients. After all, our bodies naturally produce compounds to continuously maintain and revitalize our skin. Resvita Biosciences seeks to restore and maintain the vitality of the skin by properly considering the skin microbiome as part of the human body. A harmless skin probiotic will continuously deliver the therapy that a body would to maintain skin health. Simply put, the treatment comes from the outside-in, instead of the inside-out.
Through synthetic biology and metabolic engineering, our goal is to develop a safe and versatile skin microbial platform that can address the causes of skin aging and disease. We have a unique patented approach, a rich product pipeline, clear regulatory advantages, and an experienced scientific management team. Within the next three years, we are confident that we will deliver multiple anti-aging products to market, an IND to treat an orphan skin disease with fast-track FDA designation, and exciting preclinical data to treat multiple inflammatory skin diseases like eczema and psoriasis.
Jay Keasling’s Story
A “living treatment” may ease a severe skin disease
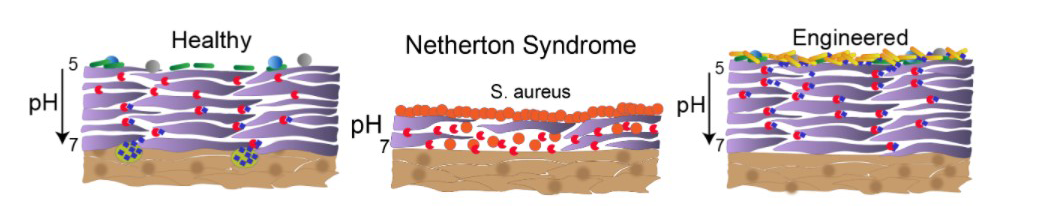
Netherton Syndrome is a rare, genetic skin disease that can be fatal to neonatal patients due to sepsis, hypothermia and dehydration. Survivors often live constantly battling flare-ups that result in painful redness and scaling of the skin throughout their bodies.

The disease affects over 1,000 people in the U.S. It is caused by a mutation in a gene for an enzyme known as LEKTI, essential for the body’s normal cycle of breaking down and replenishing skin. There is no cure.
Bioengineer Jay Keasling, PhD, aims to employ a harmless bacterium to deliver the LEKTI enzyme that Netherton children lack, restoring the natural cycle that assures healthy skin and giving them a chance for a normal life. Keasling is professor of chemical and biomolecular engineering.
His Bakar Fellows funding supports a two-year research program to engineer the bacteria to produce LEKTI; then test the protein-delivery strategy in an FDA-approved model of human skin before moving on to tests in mice.
Dr. Keasling describes the disease and his “living” treatment strategy.
Q. What happens to the skin in an infant or child with Netherton?
A. It’s an absolutely brutal disease. Normally, the epidermis sloughs off and adds new skin in about a two-week cycle. Simplistically, two classes of enzymes are involved. Enzymes called kallikreins are proteases that break down the skin, and the LEKTI enzyme, a protease inhibitor, inhibits the kallikrein activity. Without LEKTI, the epidermis breaks down, but it never builds back up.
Essentially the kids don’t have a functioning epidermis. Instead of pliant skin that maintains moisture, the scaly outer surface rapidly loses moisture and is very vulnerable to infection. The skin layer gets extremely inflamed and painful. The skin is an intense orange-red color from head to toe. It is shocking.
Q. What are current treatments for Netherton syndrome?
A. There are no FDA-approved treatments, but standard of care involves lotions, and antibiotics. Sometimes a steroidal anti-inflammatory. They can’t provide continuous relief, so at best the pain and inflammation subsides and then spikes again. Over and over.
Q. How would your proposed treatment work?
A. The bacterium with the gene for the LEKTI protein would be in a probiotic lotion applied to the skin. We can put a tag on the protein that targets it to be secreted by the microbe. It would be a living treatment. After a shower, it would just be reapplied. Ideally, it would provide slow, continuous regulation of the kallikreins. It would replenish the skin from the outside in.
Q. How do you go from a natural bacterium to a LEKTI delivery agent?
A. We genetically engineer the bacterium to produce the LEKTI protein. The microbe, Corynebacterium glutamicium, is a relative of one that lives on the skin. It is routinely bioengineered to produce amino acids for supplements and animal feed. It is designated by the FDA as GRAS – generally regarded as safe.
Q. How did you think to do this treatment strategy?
A. I’ve engineered microbes to produce a number of products. We’ve produced an antimalarial drug, artemisinin, that’s now used in Africa. A company I started produces flavors and fragrances and cosmetics using the same microbe that was engineered to produce artemisinin.
But this bacterium isn’t only a living factory for generating useful products like that. It is living on the skin. I haven’t used bioengineering for use in the microbiome.
A scientist in my laboratory, Amin Zargar, helped me conceive of the project during the early period of Covid. He went into the lab and did all of the experiments himself. He wasn’t trained in skin biology or microbiomes. He learned what he needed to learn to do the research, and along the way, he envisioned the applications for this technology.
Q. What has been your progress so far and what’s next?
A. We’ve shown that the bacterium can successfully produce LEKTI in a laboratory model of actual human skin, called EpiDerm. The Bakar Fellows support will allow us to do more research to help us fine tune it, and to move to testing it in mice for its safety and its ability to generate the enzyme.
Q. What hurdles can you envision?
A. We will need to learn to tune it – to get the right amount of protein expression. Too much may not be a good thing. And too little would not succeed. These are all doable things.
Q. Have you secured a patent for this strategy?
A. Yes. The patent is for establishing it as a platform technology to treat this and other skin disease, such as psoriasis and aging skin. We’ve just launched a startup, Revista Biosciences to help move the product to market when we’ve demonstrated its success and clinical trials confirm it is safe and effective.
I really hope that this product can make the lives of kids with Netherton a whole lot better than current treatments can.