
Tanja Cuk
ChemistryTanja Cuk graduated from Princeton University with a BSE in Electrical Engineering (2000) and Stanford University with a PhD in Applied Physics (2007). She then went on to complete a Miller Postdoctoral Fellowship at UC Berkeley (2007-2010). She joined the faculty of the Chemistry Department at UC Berkeley as an Assistant Professor in 2010. Her research involves understanding catalytic processes at solid-liquid interfaces using ultrafast optical/infrared spectroscopy and in-situ x-ray spectroscopy.
Spark Award Project
Next Generation Supercapacitors: Managing Pseudocapacitance with Metal Ions
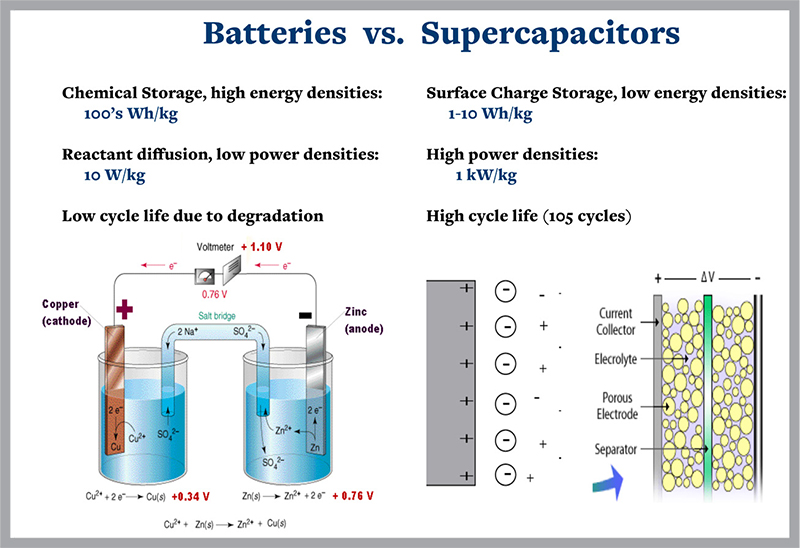
Supercapacitors store energy capacitively, as a surface charge; whereas batteries store energy chemically, via oxidation and reduction reactions. Compared to batteries, they can deliver energy much more rapidly (higher power), show enhanced reliability, and longer charge/discharge cycle lifetimes. The primary disadvantage of supercapacitors is their low energy density. It is well known that the capacitance, and hence the energy stored, can be enhanced 10-100 times by exploiting pseudocapacitance—the capacitance that arises from equilibrium charge transfer between molecules in the electrolyte and the electrode. The purpose of this Bakar fellowship proposal is to increase the number of materials that can be utilized for pseudo-capacitive behavior and explore the limits to energy density stored as a surface charge. This fundamentally new approach involves placing multiple types of metal ions in solution with transition metal oxide electrodes. The consequences will be monitored with electrochemical measurements, x-ray absorption/photoemission, scanning transmission x-ray microscopy, and optical second harmonic generation. The result will be a flexible materials system that can be optimized for performance.
Tanja Cuk’s Story
Packing Power
By: Wallace Ravven
April 22, 2013
Fast, powerful and always ready to go. Qualities that apply to good fullbacks and heavy-duty trucks are also hallmarks of the ideal electric car battery. Conventional car batteries store energy reasonably well, but other technologies can deliver energy more quickly. UC Berkeley’s Tanja Cuk (pronounced “chook”) leads an effort aimed at achieving higher energy densities in these quick technologies.
An assistant professor of chemistry, she is testing how to optimize new devices for both power delivery and energy storage. Her focus is an alternative to conventional batteries, called a “supercapacitor.”

While batteries store energy chemically, supercapacitors store it as an electrical charge which builds up at the interface between electrically charged water molecules, or ions, and an electrode. The charged water molecules move from the supercapacitor chamber to the charged metal electrode. Cuk is testing how combinations of charged metal ions, instead of only water ions, can boost capacitors’ ability to store energy on different types of electrodes.
She aims to increase supercapacitors ability to store energy ten-fold. Her lab has received new momentum in the form of a 2012 Bakar Fellowship award given to UC Berkeley scientists and engineers to help advance innovative research by early career faculty. The program focuses on projects that hold commercial promise.
Supercapacitors generate power by using surface chemical reactions interacting with an internal structure that has a high surface area. The process delivers more power than current batteries. It does so more reliably and with longer lifetimes. But as yet supercapcitors are not as good as batteries in storing energy.
“Current supercapacitors can store small amounts of energy for a long time. I’m working on technologies to store more energy, where the tradeoff might be a shorter storage time. You could use the supercapacitor when you need it for fast power delivery, like speeding up a hill, and then the battery could recharge it. It would be good for quick on-off cycles.”
Essentially, the approach can be thought of as the basis for another kind of hybrid vehicle, with the supercapacitor replacing the battery for bursts of power, and the battery recharging it when it isn’t needed. The strategy will provide a longer battery life for the car overall. Ultimately, Cuk thinks, linking supercapacitors and batteries in an electric car will offer the best of both worlds.
stored by building up a bulk material on one electrode, the supercapacitor stores energy by
creating a surface charge on two electrodes. Energy storage in the supercapacitor derives
from the extremely small distance between the electrode and the stew of ions it attracts.
The separation can be less than a millionth of an inch.
Her Bakar Fellowship supports research to devise supercapacitors that use the chemistry of mixtures of metal ions depositing on an electrode to boost the capacitance and hence, energy stored. Metal ions in a supercapcitor tend to pile up on the electrode. Cuk expects that a mixture of different types will lead the ions to deposit in a more dispersed fashion, speeding up electrical discharge at any voltage.
Cuk’s lab has already felt the impact of the Bakar support. “The focus on commercial application energizes the lab,” she says. “We have an application-oriented project.
“It’s an area I wouldn’t have gone into without this fellowship. It really complements our fundamental research and applies a focus on performance. I am now more aware of studying the tradeoffs between fast and slow energy delivery, and this helps me understand chemical reaction kinetics in a very practical way.”
She hopes to develop a proof-of concept of the capabilities of the new types of supercapacitors in the next year. Bakar support will help shepherd this prototype concept to commercialization.