
Ke Xu
ChemistryKe Xu is Assistant Professor in the Department of Chemistry and holds the Chevron Chair in Chemistry. His lab works on the development of microscopy, spectroscopy, and other physiochemical tools to visualize biological structures and nanomaterials at the nanoscale. He obtained his B.S. from Tsinghua University, Ph.D. from Caltech, and performed postdoctoral research at Harvard University before joining UC Berkeley.
Spark Award Project
Spectrally-Resolved Super-Resolution Microscopy: Redefining the Way We See the World
Microscopy, in particular light (optical) microscopy, is an indispensable tool for modern research, medical diagnosis, and quality control. The resolution of conventional optical microscopy is limited by the diffraction of light to about 300 nanometers. Emerging super-resolution microscopy methods, including our recent developments, have overcome this limit by reinventing how a light signal is generated and processed. In recent years, achieve optical resolutions of better than 10 nanometers. Beyond pursuing high spatial resolution, we are developing next-generation methods to integrate super-resolution microscopy with spectroscopy to achieve spectrally-resolved super-resolution microscopy. By exploiting the largely unexplored spectral dimension of fluorophores, our research could enable super-resolution microscopy for infinite color channels with minimal crosstalk between different channels. That could give scientists unprecedented information about the interactions between different molecular components in biological and other complex systems, thus opening up exciting new possibilities for future research and diagnostics. We also plan to make our technology work with conventional commercial microscope systems so it can be readily adapted by researchers in different fields.
Ke Xu’s Story
For 400 years curious eyes have peered into telescopes and microscopes to see what the unaided eye cannot. The invention of the light microscope in the early 17th century revolutionized how we see the world. The vivid shape, color, and dynamics revealed on the microscopic scale led to the discovery of cells and bacteria, and opened the door to modern biology.
But every instrument has its limitations. Because of the wave-like nature of light, traditional microscopes encounter an apparently insurmountable resolution limit. They can’t resolve anything smaller than half the wavelength of light — about 300 nanometers, or 1/200th diameter of a human hair.
A resolution of 300 nanometers is good enough for routine biopsies or detecting bacteria. But it can’t efficiently capture proteins — the molecules that do the real work inside cells. Proteins take on many shapes, from rod-like to globular, and they range in size form one to ten nanometers.
Proteins turn genes on or off, and haul chromosomes to opposite poles before division. They control the fate of cells. Studying their interactions could reveal defects that cause disease.
Major advances in the field of fluorescence microscopy finally brought this goal within reach. Fundamentally, fluorescence microscopy tags targets under study
with light-emitting (fluorescent) molecules to reveal their structure and activity. The emergence of a class of techniques known as super-resolution fluorescence microscopy reinvented how the fluorescence signals are generated and detected, yielding a resolution of about 10 nanometers. Proteins come into view.
The outstanding image resolution does come at a cost: the rich dimension of color, as observed with conventional light microscopy, is lost. Black-and-white images are great for looking at individual targets, but not for discerning interactions between proteins.
This year, Ke Xu, an assistant professor of chemistry and a new Bakar Fellow, developed a way to restore the missing color (more precisely, the spectrum) component in super-resolution fluorescence microscopy. Drawing on his earlier postdoctoral research with Xiaowei Zhuang at Harvard, he designed a new super-resolution microscope/spectrometer that simultaneously records the position and the spectrum of each individual fluorescence molecule in a sample.
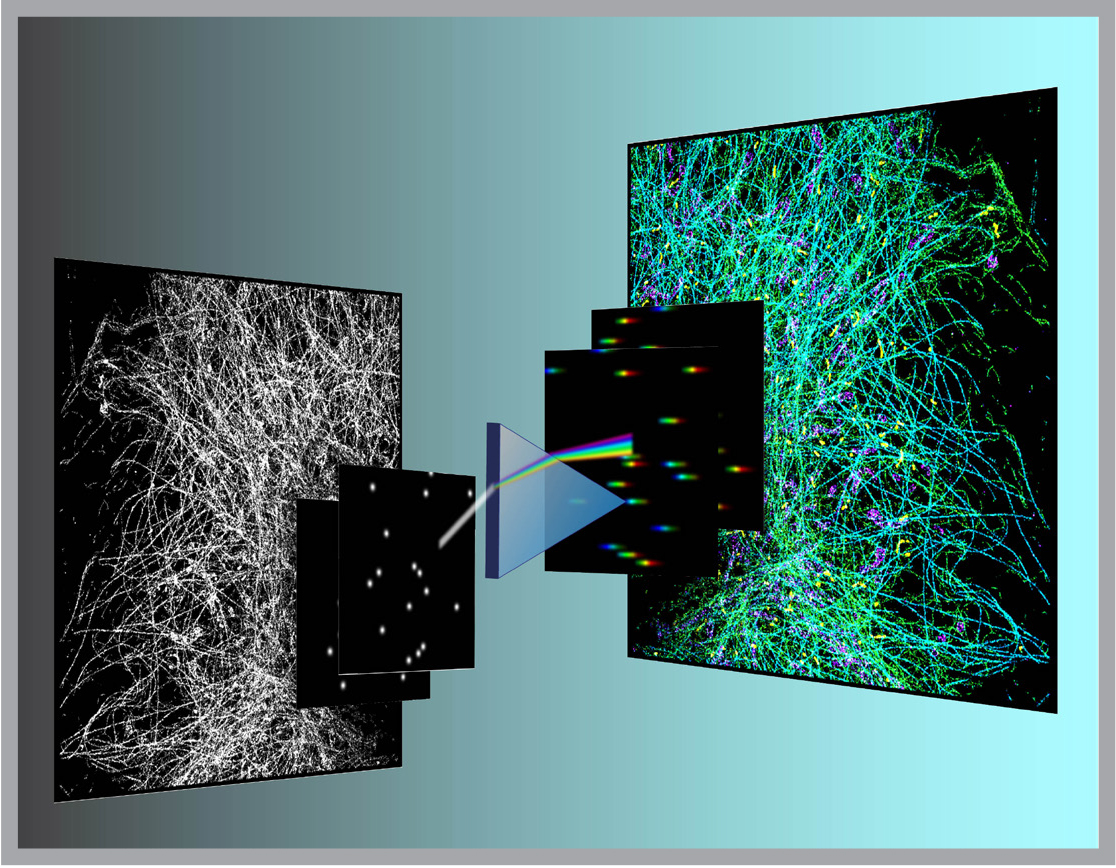
Using the new strategy Xu constructed “true-color” super-resolution images for the first time. As a first demonstration, he tagged mitochondria and three other subcellular structures, each with similar, but different fluorescent molecules. In his mages, the nuances of the four different molecules could be clearly resolved.
“This gives us the unique edge of being able to tease out the interactions between different subcellular structures in the cell based on a single image, no matter if they are organelles or pathogens,” he says.
Supported by the Bakar Fellows Program, he aims to advance this powerful imaging capacity and adapt it to the kinds of microscopes currently in use in labs around the world.
“We need to build a black box that works with existing microscopes. It must be about a tenth the current size
and much easier for non-specialists to use,” he says. “If we can do that, scientists will be able to study the internal evolution of cells at a more meaningful level.”
He is zeroing in on the proteins that drive cell division. In a process that is still only partly understood, protein interactions along mini tracks called microtubules pull sister chromosomes to opposite poles before a cell divides. Xu’s lab uses the new imaging system to examine the protein interplay that underlie this fundamental step in growth. A better understanding, he says, may provide clues to certain disorders of cell division, including the out-of-control duplication of cancer cells.
He has filed for a patent for his imaging innovations, and is considering launching a startup to promote use of the powerful new system.
“Berkeley colleagues who have already been Bakar Fellows for several years have been very generous, coaching me about building a startup and licensing my technology,” he says.
Bakar Fellows mentors are also helping him approach microscope manufacturers to demonstrate the commercial potential of the increased imaging power and its ability to shed light on what literally drives life.